Home /
Expert Answers /
Chemistry /
given-the-equilibrium-constants-for-the-following-reactions-4cu-s-o-2-g-2cu-2-o-s-k-1-9-7-pa756
(Solved): Given the equilibrium constants for the following reactions: 4Cu(s) O_(2)(g)2Cu_(2)O(s),K_(1)=9.7 ...
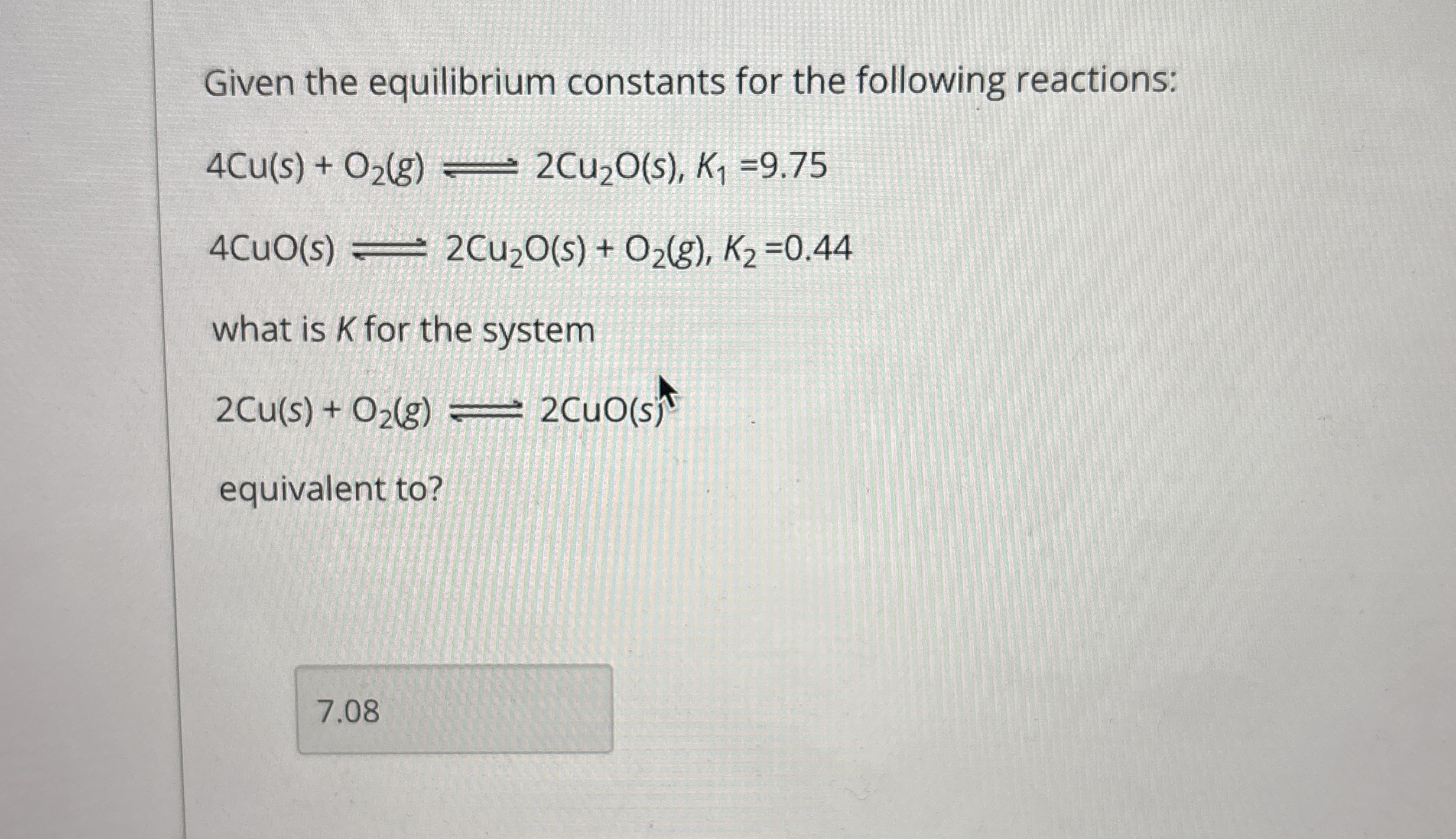
Given the equilibrium constants for the following reactions:
4Cu(s) O_(2)(g)⇌2Cu_(2)O(s),K_(1)=9.75
4CuO(s)⇌2Cu_(2)O(s) O_(2)(g),K_(2)=0.44what is
Kfor the system
2Cu(s) O_(2)(g)⇌2CuO(s)equivalent to?