Home /
Expert Answers /
Chemistry /
0-question-2-1-pts-a-chemical-reaction-was-carried-out-using-36-8-ml-of-1-25-m-hydrochloric-acid-h-pa797
(Solved): 0 Question 2 1 pts A chemical reaction was carried out using 36.8 mL of 1.25 M hydrochloric acid. H ...
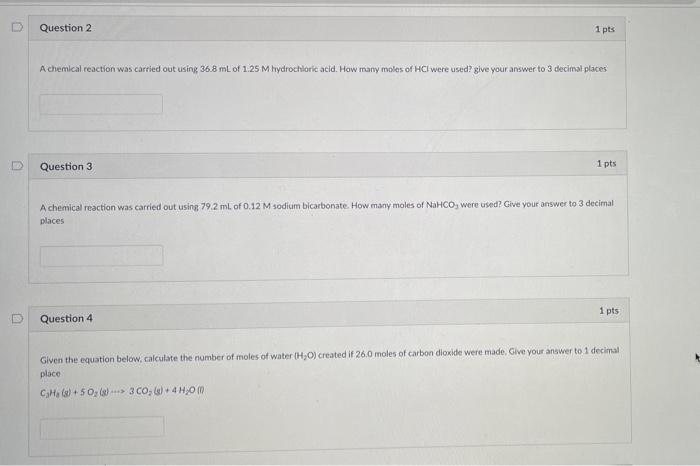
0 Question 2 1 pts A chemical reaction was carried out using 36.8 mL of 1.25 M hydrochloric acid. How many moles of HCI were used? give your answer to 3 decimal places Question 3. 1 pts A chemical reaction was carried out using 79.2 ml of 0.12 M sodium bicarbonate. How many moles of NaHCO, were used? Give your answer to 3 decimal places 1 pts Question 4 Given the equation below, calculate the number of moles of water (H?O) created if 26.0 moles of carbon dioxide were made. Give your answer to 1 decimal place CaHa (8)+50? (8) 3 CO? (g) + 4H?Om
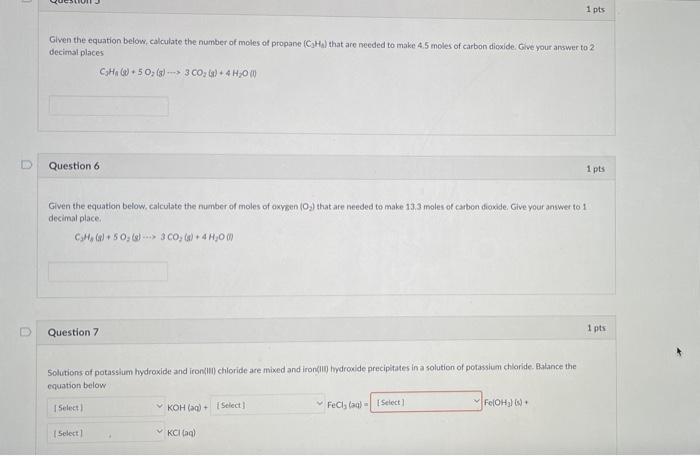
1 pts Given the equation below, calculate the number of moles of propane (C?Ha) that are needed to make 4.5 moles of carbon dioxide. Give your answer to 2 decimal places C?Ha (+50? (3) 3 CO? (g) + 4H?0 (1) Question 6 1 pts Given the equation below, calculate the number of moles of oxygen (O?) that are needed to make 13.3 moles of carbon dioxide. Give your answer to 11 decimal place.. CH? (8) + 5O?(g) 3 CO? (s) + 4H?O (1) Question 7 1 pts Solutions of potassium hydroxide and iron(III) chloride are mixed and iron(III) hydroxide precipitates in a solution of potassium chloride. Balance the equation below. [Select] KOH(aq) + [Select] FeCl, lag) [Select) Fe(OH?) (s) + [Select] ?KCI (aq)