Home /
Expert Answers /
Chemistry /
1-consider-three-different-ionic-compounds-all-of-which-have-a-cation-with-a-charge-of-1-a-pa342
(Solved): 1. Consider three different ionic compounds, all of which have a cation with a charge of \( +1 \) a ...
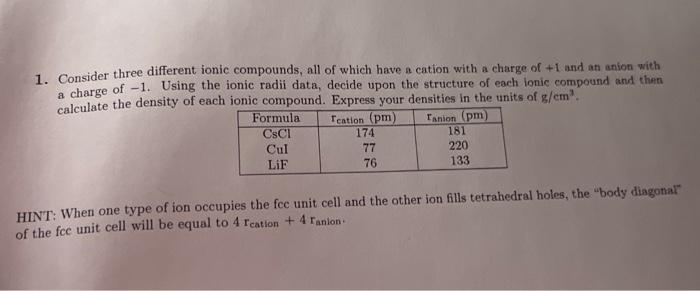
1. Consider three different ionic compounds, all of which have a cation with a charge of \( +1 \) and an anion with a charge of \( -1 \). Using the ionic radii data, decide upon the structure of each ionic compound and than calculate the density of each ionic compound. Express your densities in the units of \( \mathrm{g} / \mathrm{cm}^{3} \). HINT: When one type of ion occupies the fce unit cell and the other ion flls tetrahedral holes, the "body diagonal" of the fce unit cell will be equal to \( 4 r_{\text {cation }}+4 r_{\text {anlon. }} \).