Home /
Expert Answers /
Chemistry /
1-for-the-following-reaction-at-25c-an-experiment-starting-with-a-0-210m-concentration-of-a-pa187
(Solved): (1) For the following reaction at 25C, an experiment starting with a 0.210M concentration of A ...
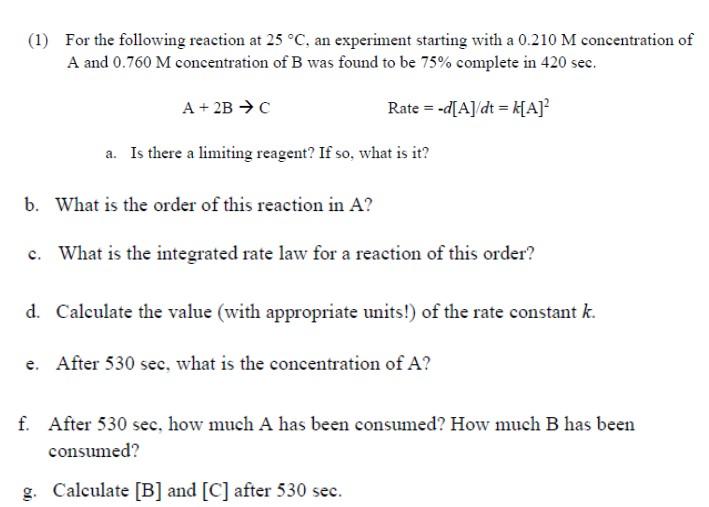
(1) For the following reaction at , an experiment starting with a concentration of and concentration of B was found to be complete in . a. Is there a limiting reagent? If so, what is it? b. What is the order of this reaction in ? c. What is the integrated rate law for a reaction of this order? d. Calculate the value (with appropriate units!) of the rate constant . e. After , what is the concentration of ? f. After 530 sec, how much A has been consumed? How much B has been consumed? g. Calculate and after .
Expert Answer
a. To determine whether there is a limiting reagent, moles of A = concentration of A x volume of solution = 0.210 mol/L x 1 L = 0.210 molmoles of B =