Home /
Expert Answers /
Mechanical Engineering /
1-mathrm-m-3-of-air-is-heated-reversibly-at-constant-pressure-from-15-circ-mathr-pa804
(Solved): \( 1 \mathrm{~m}^{3} \) of air is heated reversibly at constant pressure from \( 15^{\circ} \mathr ...
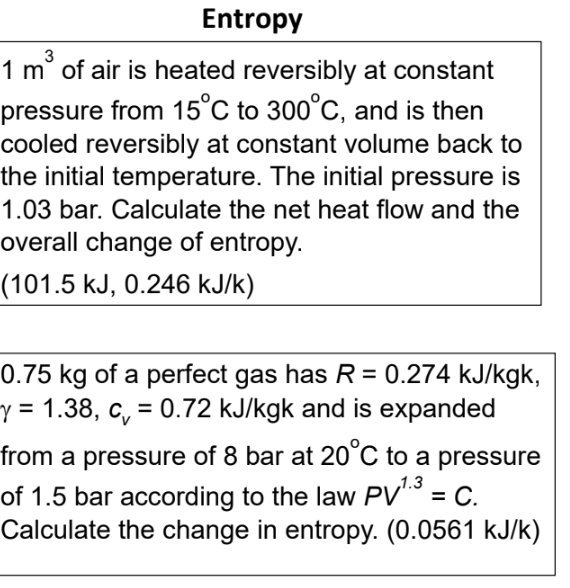
\( 1 \mathrm{~m}^{3} \) of air is heated reversibly at constant pressure from \( 15^{\circ} \mathrm{C} \) to \( 300^{\circ} \mathrm{C} \), and is then cooled reversibly at constant volume back to the initial temperature. The initial pressure is \( 1.03 \) bar. Calculate the net heat flow and the overall change of entropy. \( (101.5 \mathrm{~kJ}, 0.246 \mathrm{~kJ} / \mathrm{k}) \) \( 0.75 \mathrm{~kg} \) of a perfect gas has \( R=0.274 \mathrm{~kJ} / \mathrm{kgk} \), \( \gamma=1.38, c_{v}=0.72 \mathrm{~kJ} / \mathrm{kgk} \) and is expanded from a pressure of 8 bar at \( 20^{\circ} \mathrm{C} \) to a pressure of \( 1.5 \) bar according to the law \( P V^{1.3}=C \). Calculate the change in entropy. \( (0.0561 \mathrm{~kJ} / \mathrm{k}) \)