Home /
Expert Answers /
Chemistry /
1-what-happens-if-o3-g-is-removed-from-equilibrium-in-the-following-reaction-3o2-g-lt-gt-2o-pa139
(Solved): 1. What happens if O3 (g) is removed from equilibrium in the following reaction? 3O2 (g) <> 2O ...
1. What happens if O3 (g) is removed from equilibrium in the following reaction? 3O2 (g) <> 2O3 (g)
a) The equilibrium shifts towards products.
b) The equilibrium shifts toward the reactants.
c) The equilibrium does not change
d) balance is not restored
2. In what direction will the equilibrium shift if the volume of the following system is increased? N2O4 (g) <> 2NO (g)?
a) Equilibrium shifts towards products
b) The equilibrium shifts toward the reactants.
c) The equilibrium does not change
d) balance is not restored
3. What would happen to the concentration of iodine bromide if the volume is reduced? I2 (g) + Br2 (g) <> 2IBr (g)
a) Decreases
b) Increases
c) the reaction stops
d) does not change
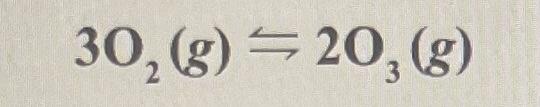
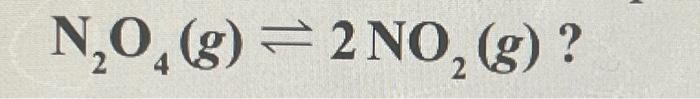
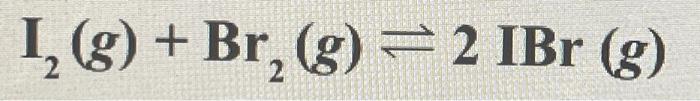
30, (g) = 20, (g)
N?O?(g) = 2 NO? (g) ?
I? (g) + Br? (g) = 2 IBr (g) 2