Home /
Expert Answers /
Chemistry /
1-write-a-balanced-molecular-equation-and-net-ionic-equation-for-the-precipitation-of-calcium-carbon-pa785
(Solved): 1.Write a balanced molecular equation and net-ionic equation for the precipitation of calcium carbon ...
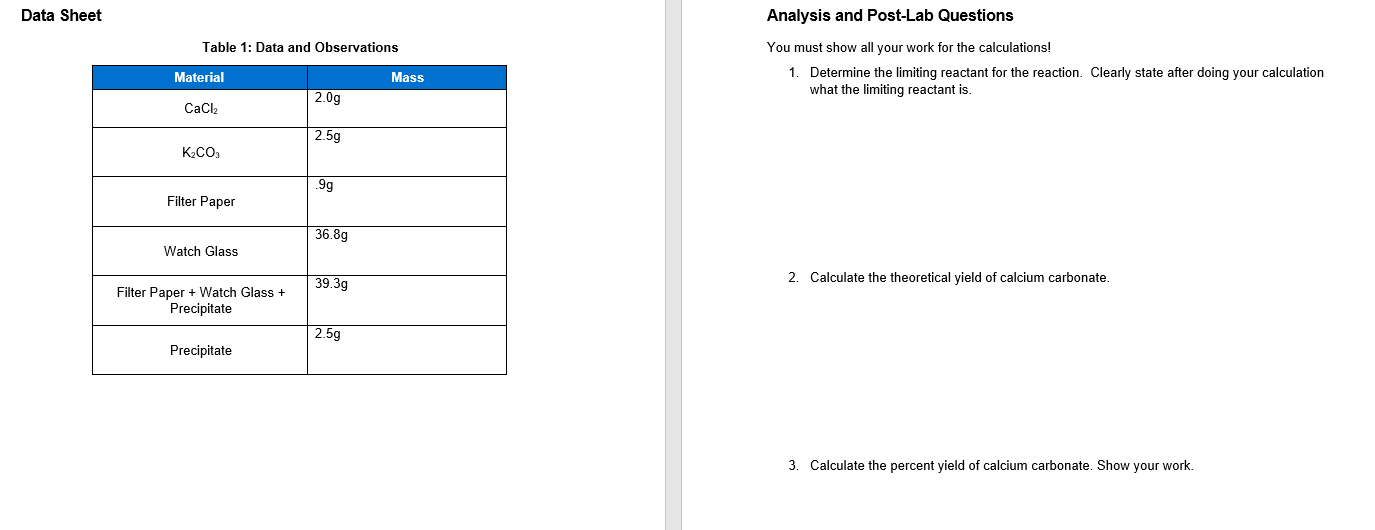
1.Write a balanced molecular equation and net-ionic equation for the precipitation of calcium carbonate from potassium carbonate and calcium chloride

Data Sheet Analysis and Post-Lab Questions Tahle 1: Data and Ohservations You must show all your work for the calculations! 1. Determine the limiting reactant for the reaction. Clearly state after doing your calculation what the limiting reactant is. 2. Calculate the theoretical yield of calcium carbonate. 3. Calculate the percent yield of calcium carbonate. Show your work.
4. Comment on your percent yield and discuss possible reasons specific to the reaction or experimental procedure that your percent yield is not \( 100 \% \)