Home /
Expert Answers /
Chemistry /
13-11-the-equilibrium-constant-for-the-conjugate-acid-base-pair-hin-h-2-oh-3-o-in-is-8-pa945
(Solved): 13-11 The equilibrium constant for the conjugate acid-base pair HIn H_(2)OH_(3)O^( ) In^(-) is 8. ...
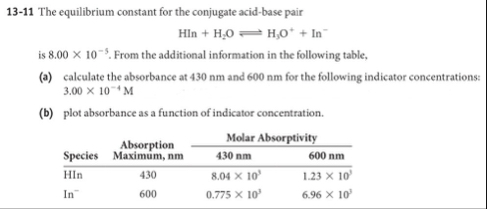
13-11 The equilibrium constant for the conjugate acid-base pair
HIn H_(2)O⇌H_(3)O^( ) In^(-)is
8.00\times 10^(-5). From the additional information in the following table, (a) calculate the absorbance at 430 nm and 600 nm for the following indicator concentrations:
3.00\times 10^(-4)M(b) plot absorbance as a function of indicator concentration. \table[[,\table[[Absorption],[Species]],Molar Absorptivity],[Maximum, nm,430 nm,600 nm,],[HIn,430,
8.04\times 10^(3),
1.23\times 10^(3)