Home /
Expert Answers /
Chemistry /
2-b-when-a-solid-dissolves-in-water-heat-evolved-or-absorbed-known-as-the-heat-of-dissolution-c-pa920
(Solved): 2. b. When a solid dissolves in water heat evolved or absorbed, known as the heat of dissolution, c ...
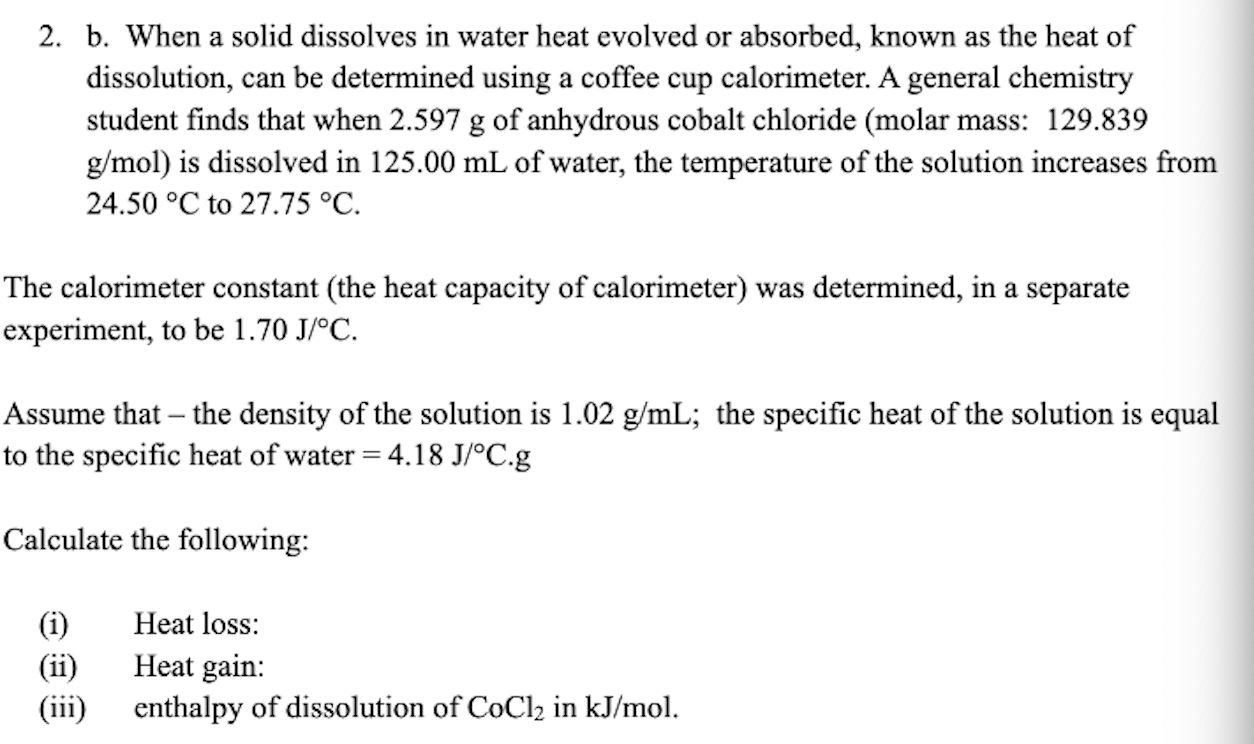
2. b. When a solid dissolves in water heat evolved or absorbed, known as the heat of dissolution, can be determined using a coffee cup calorimeter. A general chemistry student finds that when of anhydrous cobalt chloride (molar mass: 129.839 ) is dissolved in of water, the temperature of the solution increases from to . The calorimeter constant (the heat capacity of calorimeter) was determined, in a separate experiment, to be . Assume that - the density of the solution is ; the specific heat of the solution is equal to the specific heat of water Calculate the following: (i) Heat loss: (ii) Heat gain: (iii) enthalpy of dissolution of in .