Home /
Expert Answers /
Chemistry /
2-consider-the-following-reaction-a-propose-a-mechanism-using-curved-arrows-to-show-the-flow-of-pa760
(Solved): 2) Consider the following reaction: a) Propose a mechanism using curved arrows to show the flow of ...
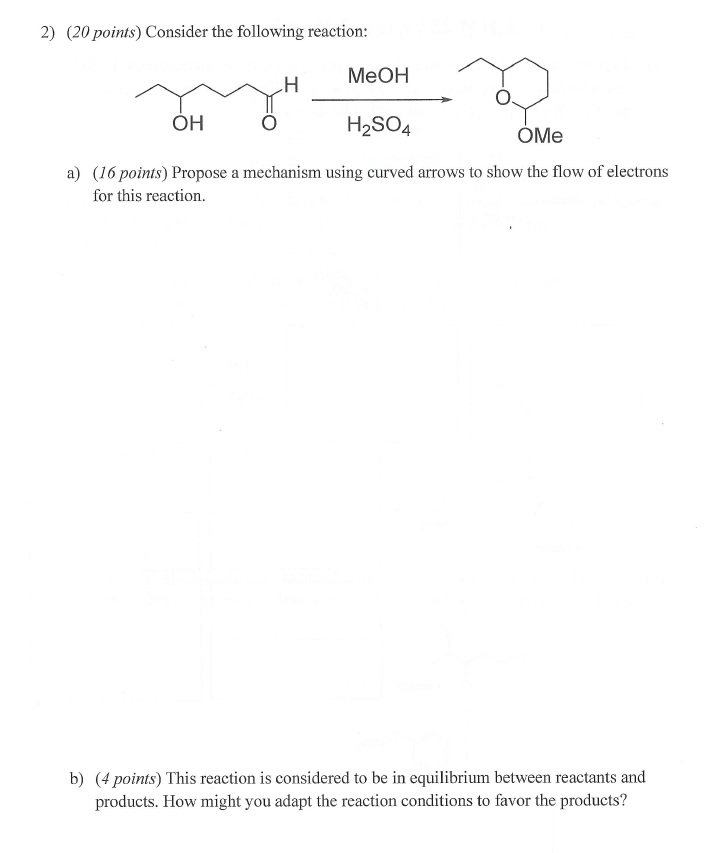
2) Consider the following reaction: a) Propose a mechanism using curved arrows to show the flow of electrons for this reaction. b) This reaction is considered to be in equilibrium between reactants and products. How might you adapt the reaction conditions to favor the products?