Home /
Expert Answers /
Chemistry /
26-a-buffer-solution-contains-0-86mol-of-phenol-hc6h5o-and-0-88mol-of-sodium-phenoxide-pa520
(Solved): #26 A buffer solution contains 0.86mol of phenol (HC6H5O) and 0.88mol of sodium phenoxide ...
#26
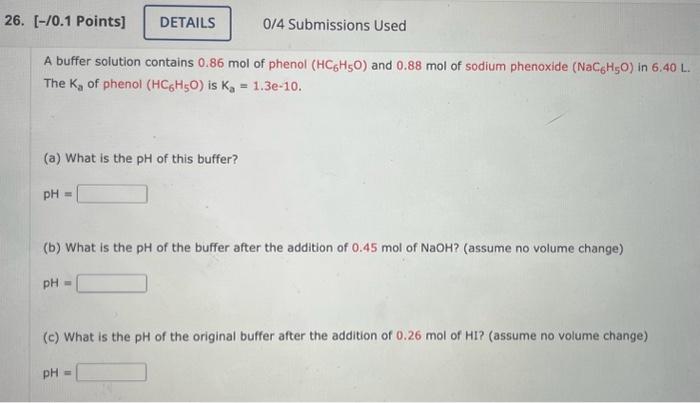

A buffer solution contains of phenol and of sodium phenoxide in . The of phenol is . (a) What is the of this buffer? (b) What is the of the buffer after the addition of of ? (assume no volume change) (c) What is the of the original buffer after the addition of of ? (assume no volume change)