Home /
Expert Answers /
Chemistry /
3-a-10-067-g-sample-of-cereal-is-placed-in-a-250-ml-erlenmeyer-flask-along-with-1-g-of-sodium-ascor-pa795
(Solved): 3. A 10.067 g sample of cereal is placed in a 250-mL Erlenmeyer flask along with 1-g of sodium ascor ...
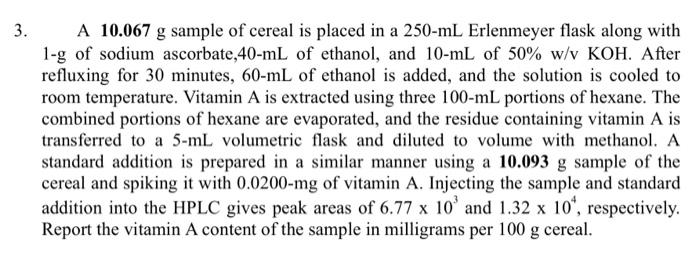
3. A 10.067 g sample of cereal is placed in a 250-mL Erlenmeyer flask along with 1-g of sodium ascorbate,40-mL of ethanol, and 10-mL of 50% w/v KOH. After refluxing for 30 minutes, 60-mL of ethanol is added, and the solution is cooled to room temperature. Vitamin A is extracted using three 100-mL portions of hexane. The combined portions of hexane are evaporated, and the residue containing vitamin A is transferred to a 5-mL volumetric flask and diluted to volume with methanol. A standard addition is prepared in a similar manner using a 10.093 g sample of the cereal and spiking it with 0.0200-mg of vitamin A. Injecting the sample and standard addition into the HPLC gives peak areas of 6.77 x 10³ and 1.32 x 10¹, respectively. Report the vitamin A content of the sample in milligrams per 100 g cereal.
3. A sample of cereal is placed in a Erlenmeyer flask along with 1 -g of sodium ascorbate, of ethanol, and of . After refluxing for 30 minutes, of ethanol is added, and the solution is cooled to room temperature. Vitamin A is extracted using three portions of hexane. The combined portions of hexane are evaporated, and the residue containing vitamin is transferred to a volumetric flask and diluted to volume with methanol. A standard addition is prepared in a similar manner using a sample of the cereal and spiking it with of vitamin A. Injecting the sample and standard addition into the HPLC gives peak areas of and , respectively. Report the vitamin A content of the sample in milligrams per cereal.