Home /
Expert Answers /
Chemical Engineering /
3-at-0-01-c-triple-point-ahvap-of-ho-is-45-kj-mol-and-ahfus-of-ice-is-6-kj-mol-a-find-ahsub-pa776
(Solved): 3.At 0.01 C (Triple point) AHvap of HO is 45 kJ/mol and AHfus of ice is 6 kJ/mol. a) Find AHsub ...
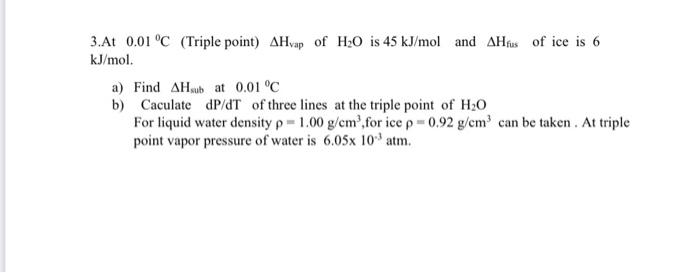
3.At 0.01 °C (Triple point) AHvap of H₂O is 45 kJ/mol and AHfus of ice is 6 kJ/mol. a) Find AHsub at 0.01 °C b) Caculate dP/dT of three lines at the triple point of H₂O For liquid water density p= 1.00 g/cm³,for ice p = 0.92 g/cm³ can be taken. At triple point vapor pressure of water is 6.05x 10-³ atm.
3.At (Triple point) of is and of ice is 6 . a) Find at b) Caculate of three lines at the triple point of For liquid water density , for ice can be taken. At triple point vapor pressure of water is .
Expert Answer
a) To find AHsub at (the triple point), we need to consider the enthalpy change associated with the sublimation of water from ice to vapor. The enthalpy change for sublimation, AHsub, can be calculated by summing the enthalpy changes for melting (...