Home /
Expert Answers /
Chemistry /
3-calculate-delta-mathrm-h-and-delta-mathrm-s-for-the-irreversible-isothermal-ch-pa677
(Solved): 3. Calculate \( \Delta \mathrm{H} \) and \( \Delta \mathrm{S} \) for the irreversible isothermal ch ...
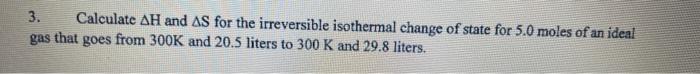
3. Calculate \( \Delta \mathrm{H} \) and \( \Delta \mathrm{S} \) for the irreversible isothermal change of state for \( 5.0 \) moles of an ideal gas that goes from \( 300 \mathrm{~K} \) and \( 20.5 \) liters to \( 300 \mathrm{~K} \) and \( 29.8 \) liters.
Expert Answer
Since ?H=2.303nRT log(vf/Vi) here n=5.0 mol T=30