Home /
Expert Answers /
Chemistry /
3-given-the-balanced-equation-below-mathrm-co-2-4-mathrm-h-2-text-mathrm-ch-pa619
(Solved): 3. Given the balanced equation below, \[ \mathrm{CO}_{2}+4 \mathrm{H}_{2} \text { } \mathrm{CH}_ ...
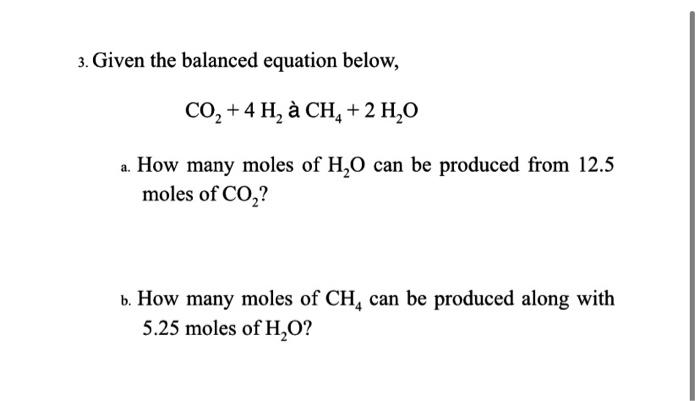
3. Given the balanced equation below, \[ \mathrm{CO}_{2}+4 \mathrm{H}_{2} \text { à } \mathrm{CH}_{4}+2 \mathrm{H}_{2} \mathrm{O} \] a. How many moles of \( \mathrm{H}_{2} \mathrm{O} \) can be produced from \( 12.5 \) moles of \( \mathrm{CO}_{2} \) ? b. How many moles of \( \mathrm{CH}_{4} \) can be produced along with \( 5.25 \) moles of \( \mathrm{H}_{2} \mathrm{O} \) ?