Home /
Expert Answers /
Chemistry /
35-an-enzyme-reaction-to-be-studied-at-ph-4-0-can-best-be-carried-out-using-a-buffer-solution-mad-pa205
(Solved): 35. An enzyme reaction to be studied at pH 4.0 can best be carried out using a buffer solution mad ...
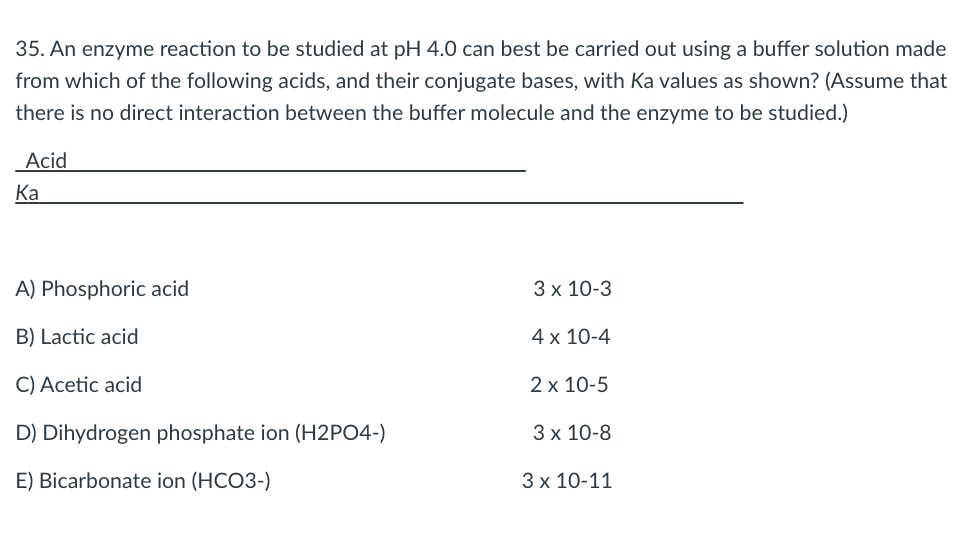
35. An enzyme reaction to be studied at pH 4.0 can best be carried out using a buffer solution made from which of the following acids, and their conjugate bases, with Ka values as shown? (Assume that there is no direct interaction between the buffer molecule and the enzyme to be studied.) Acid Ka A) Phosphoric acid 3 x 10-3 B) Lactic acid 4 x 10-4 C) Acetic acid 2 x 10-5 D) Dihydrogen phosphate ion (H2PO4-) 3 x 10-8 E) Bicarbonate ion (HCO3-) 3 x 10-11
Expert Answer
pH of a buffer is found using the equation known as the Henderson-Hasselbalch equation: pH = pK? + log([A?]/[HA])