Home /
Expert Answers /
Chemical Engineering /
4-apply-a-olscience-com-instances-33279-experimentation-419230-exploration-80668-pagenum-2-3-calc-pa893
(Solved): 4. Apply A olscience.com/instances/33279#/experimentation/419230/exploration/80668?pageNum=2 3. Calc ...
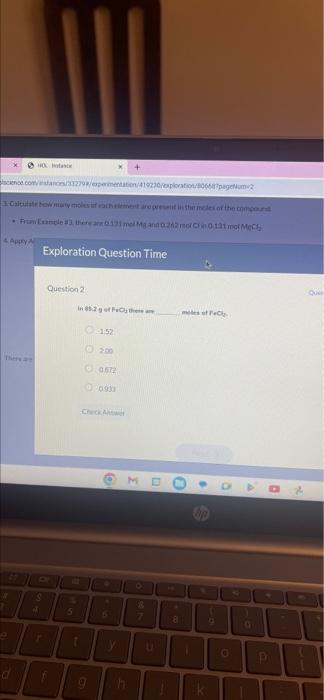
4. Apply A olscience.com/instances/33279#/experimentation/419230/exploration/80668?pageNum=2 3. Calculate how many moles of each element are present in the moles of the compound. • From Example #3, there are 0.131 mol Mg and 0.262 mol Cl in 0.131 mol MgCl₂. There are # 3 HOL- Instance d 4 Question 2 Exploration Question Time f do 5 % t In 85.2 g of FeCl2 there are 1.52 g 2.00 X 0.672 0.933 Check Answer OI + 6 y h M & 7 u 8 تا moles of FeCl2. hp k 9 0 Ques +
meles of 200 0.572 cols