Home /
Expert Answers /
Chemistry /
4-at-a-particular-temperature-k-2-0106-for-the-reaction-2co2-g-2co-g-o2-g-if-pa334
Expert Answer
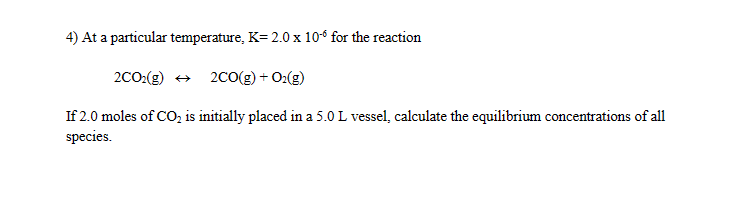
2COA2(g)?2CO(g)+OA2(g)Initial moles of COA2