Home /
Expert Answers /
Mechanical Engineering /
4-consider-complete-combustion-of-1kmol-of-pentane-gas-c5h12-with-40-excess-air-both-at-pa265
(Solved): 4. Consider complete combustion of 1kmol of pentane gas (C5H12) with 40% excess air (both at ...
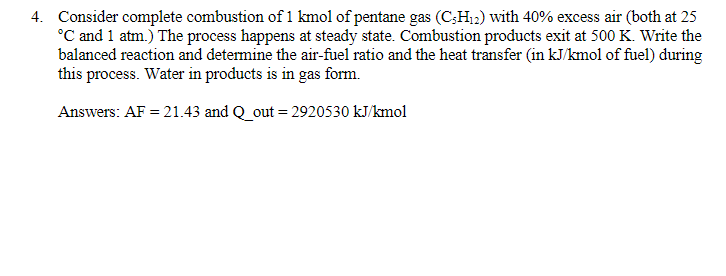
4. Consider complete combustion of of pentane gas with excess air (both at 25 and .) The process happens at steady state. Combustion products exit at . Write the balanced reaction and determine the air-fuel ratio and the heat transfer (in of fuel) during this process. Water in products is in gas form. Answers: and out
Expert Answer
The equation of combustion of pentane gas is given as, . 72 moles of pentane + 256 moles of O2 gives 220 moles of CO2 And 108 moles of water vapour.Or