Home /
Expert Answers /
Chemistry /
4-the-benzene-anomaly-cyclohexane-c6h12-cyclohexene-c6h10-1-3-cyclohexadiene-c6-pa382
(Solved): 4) The benzene anomaly. Cyclohexane (C6H12), cyclohexene (C6H10),1,3-cyclohexadiene (C6 ...
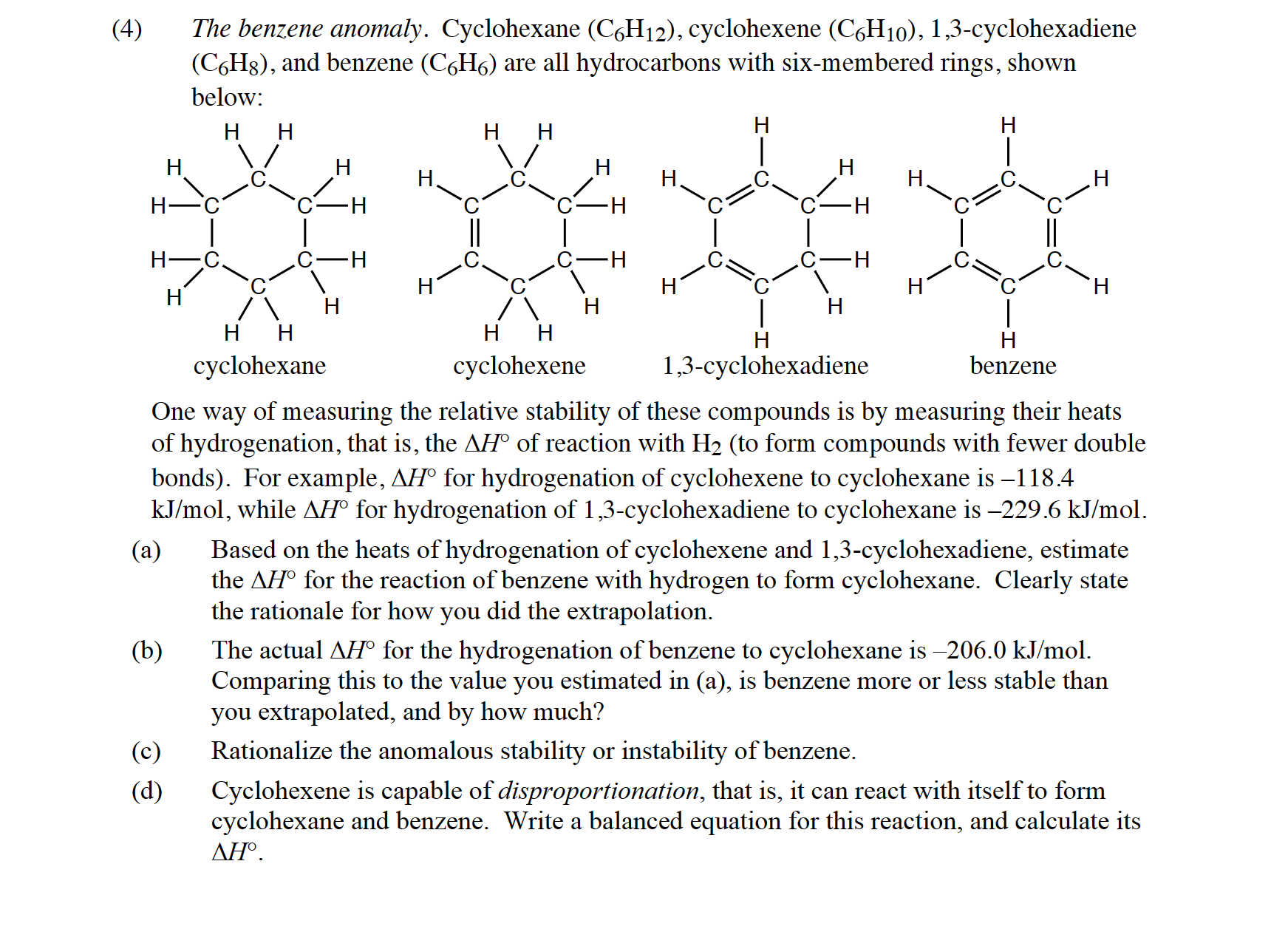
4) The benzene anomaly. Cyclohexane , cyclohexene -cyclohexadiene , and benzene are all hydrocarbons with six-membered rings, shown below: One way of measuring the relative stability of these compounds is by measuring their heats of hydrogenation, that is, the of reaction with (to form compounds with fewer double bonds). For example, for hydrogenation of cyclohexene to cyclohexane is -118.4 , while for hydrogenation of 1,3-cyclohexadiene to cyclohexane is . (a) Based on the heats of hydrogenation of cyclohexene and 1,3-cyclohexadiene, estimate the for the reaction of benzene with hydrogen to form cyclohexane. Clearly state the rationale for how you did the extrapolation. (b) The actual for the hydrogenation of benzene to cyclohexane is . Comparing this to the value you estimated in (a), is benzene more or less stable than you extrapolated, and by how much? (c) Rationalize the anomalous stability or instability of benzene. (d) Cyclohexene is capable of disproportionation, that is, it can react with itself to form cyclohexane and benzene. Write a balanced equation for this reaction, and calculate its .