Home /
Expert Answers /
Chemistry /
7-quad-1point-calculate-the-ph-at-which-the-y-carboxyl-group-of-glutamic-acid-is-two-thirds-pa250
(Solved): \( 7 \quad \) 1point Calculate the pH at which the y carboxyl group of glutamic acid is two-thirds ...
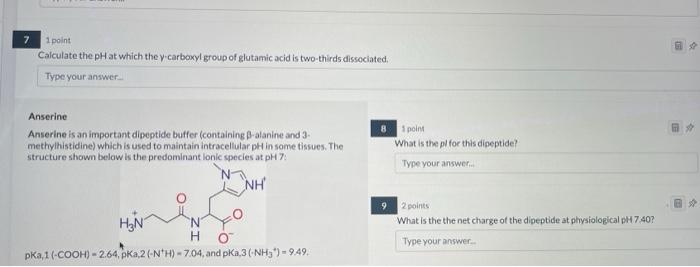
\( 7 \quad \) 1point Calculate the pH at which the y carboxyl group of glutamic acid is two-thirds dissociated. Type your answer_ Anserine Anserine is an important dipeptide buffer (containing \( \beta \)-alanine and 3 - 8 1 poins methylhistidine) which is used to maintain intracellular pH in some tissues. The What is the pif for this dipeptide? structure shown below is the predominant lonic species at pH 7 : 92 goints What is the the net charge of the dipeptide at phivsiological pH 7,40? pKa,1 \( (-\mathrm{COOH})=2.64, \mathrm{pKa}, 2\left(-\mathrm{N}^{+} \mathrm{H}\right)=7.04 \), and \( \mathrm{pKa}, 3\left(-\mathrm{NH}_{3}^{*}\right)=9.49 \)