Home /
Expert Answers /
Chemistry /
7000j-of-heat-are-added-to-a-system-the-surroundings-do-8400j-of-work-on-system-what-is-e-i-pa866
(Solved): 7000J of heat are added to a system. The surroundings do 8400J of work on system. What is E i ...
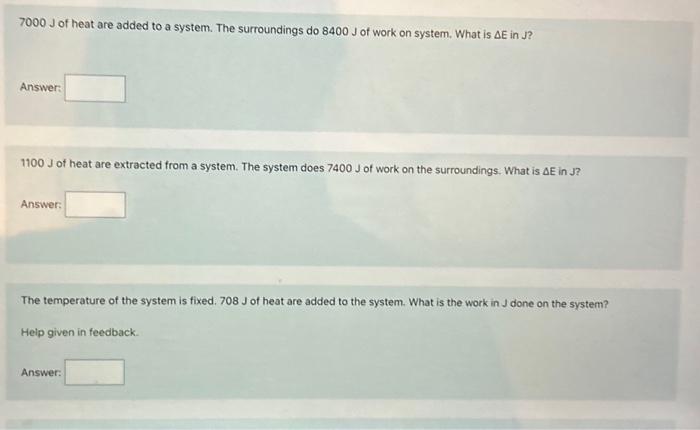
of heat are added to a system. The surroundings do of work on system. What is in J? Answer: of heat are extracted from a system. The system does of work on the surroundings. What is in J? Answer: The temperature of the system is fixed. of heat are added to the system. What is the work in J done on the system? Heip given in feedback. Answer: