(Solved): 8.5 Consider the task of analyzing the performance of a tubular J. M. Sullivan and A. E. Axworthy [ ...
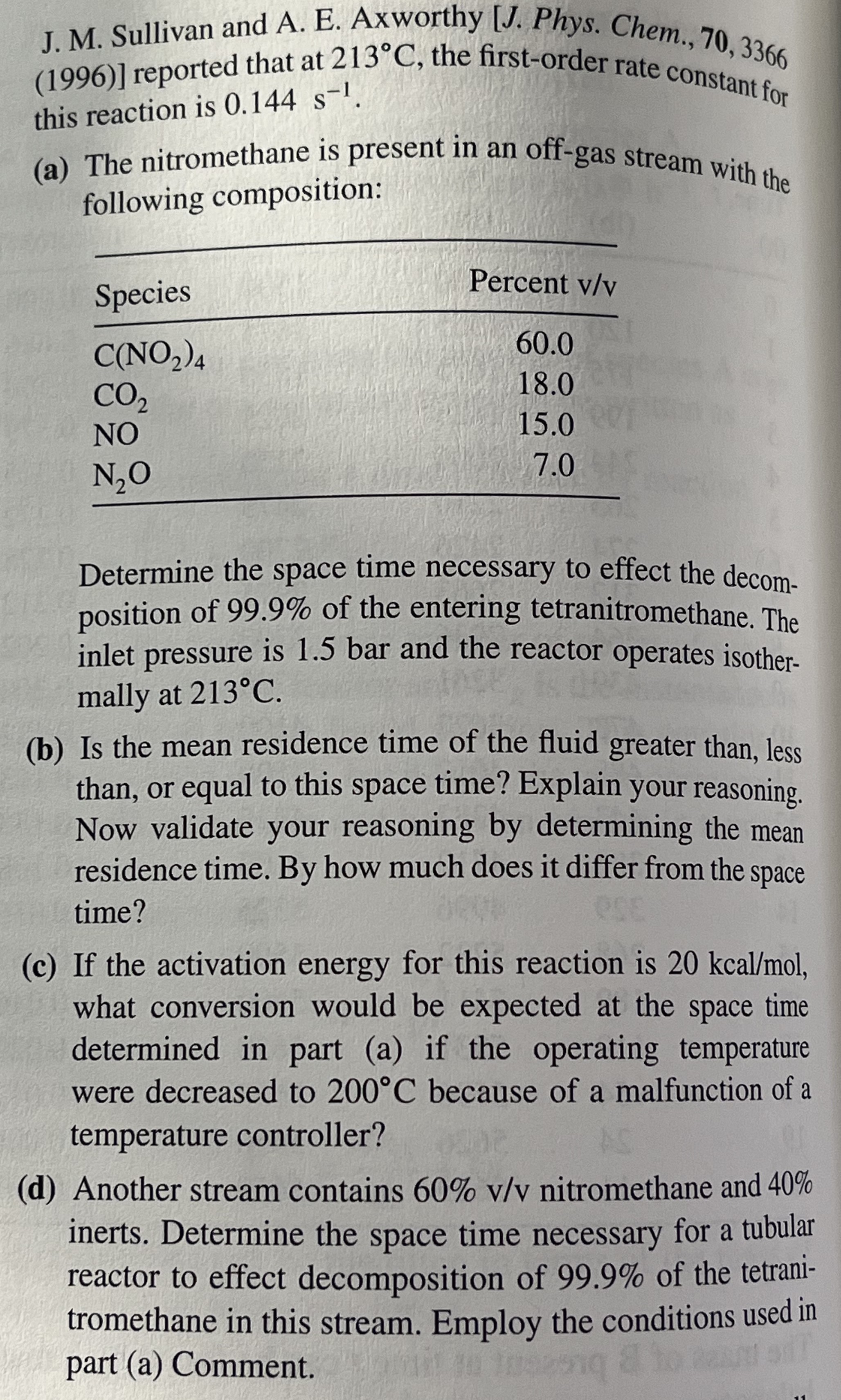
8.5 Consider the task of analyzing the performance of a tubular J. M. Sullivan and A. E. Axworthy [J. Phys. Chem., 70, 3366 (1996)] reported that at
213\deg C, the first-order rate constant for this reaction is
0.144s^(-1). (a) The nitromethane is present in an off-gas stream with the following composition: Determine the space time necessary to effect the decom- position of
99.9%of the entering tetranitromethane. The inlet pressure is 1.5 bar and the reactor operates isother- mally at
213\deg C. (b) Is the mean residence time of the fluid greater than, less than, or equal to this space time? Explain your reasoning. Now validate your reasoning by determining the mean residence time. By how much does it differ from the space time? (c) If the activation energy for this reaction is
20kca(l)/(m)ol, what conversion would be expected at the space time determined in part (a) if the operating temperature were decreased to
200\deg Cbecause of a malfunction of a temperature controller? (d) Another stream contains
60%(v)/(v)nitromethane and
40%inerts. Determine the space time necessary for a tubular reactor to effect decomposition of
99.9%of the tetrani- tromethane in this stream. Employ the conditions used in part (a) Comment. reactor in which decomposition of tetranitromethane is taking place at steady state. The stoichiometry of the reaction is
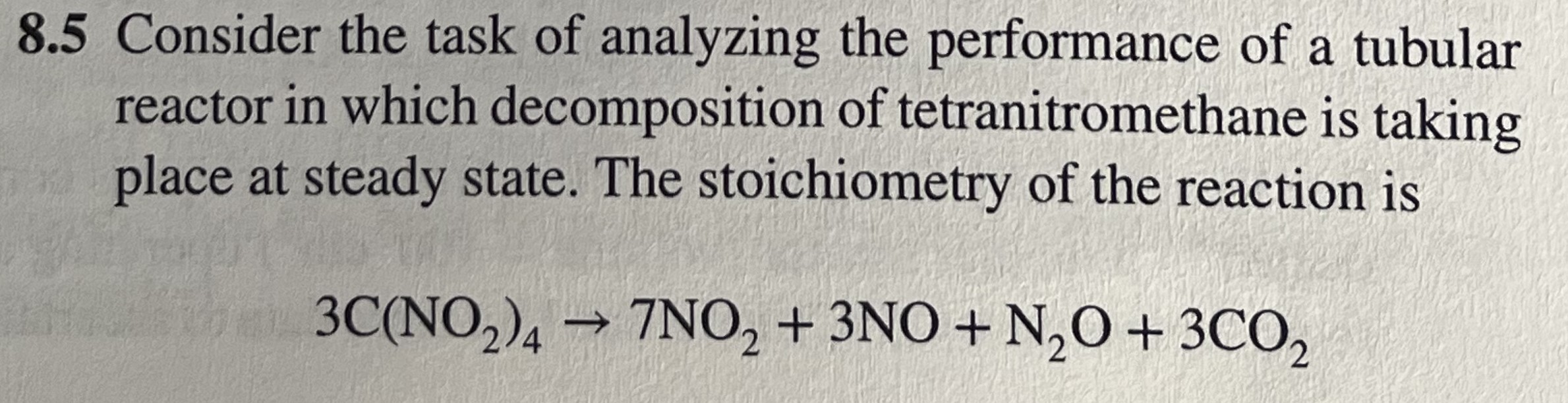
3C(NO_(2))_(4)->7NO_(2)+3NO+N_(2)O+3CO_(2)