Home /
Expert Answers /
Chemistry /
8-a-0-37m-solution-of-a-weak-base-b-has-a-ph-of-9-12-the-equation-for-ioniz-pa550
Expert Answer
The equation for the ionization of B is given
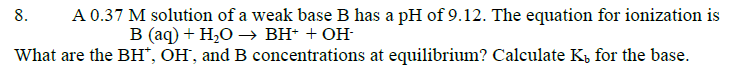 ???????
???????