Home /
Expert Answers /
Chemistry /
a-0-239-mathrm-g-sample-of-a-gas-in-a-100-mathrm-ml-flask-exerts-a-pressure-of-pa647
(Solved): A \( 0.239 \mathrm{~g} \) sample of a gas in a \( 100-\mathrm{mL} \) flask exerts a pressure of \( ...
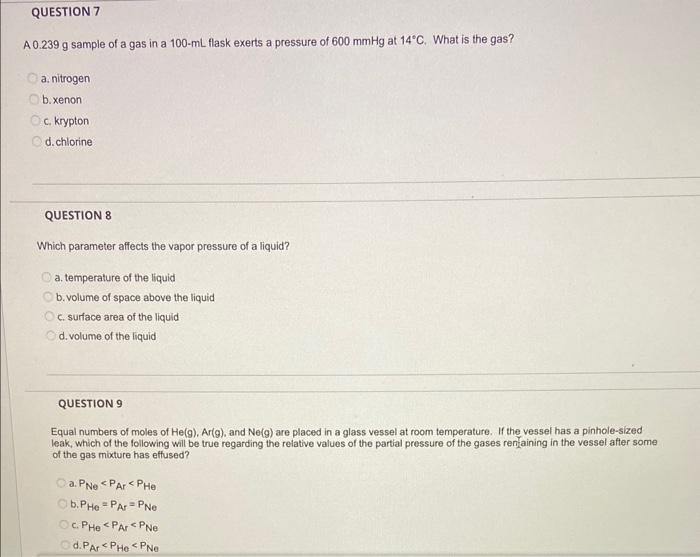
A \( 0.239 \mathrm{~g} \) sample of a gas in a \( 100-\mathrm{mL} \) flask exerts a pressure of \( 600 \mathrm{mmHg} \) at \( 14^{\circ} \mathrm{C} \). What is the gas? a. nitrogen b. xenon c. krypton d. chlorine QUESTION 8 Which parameter affects the vapor pressure of a liquid? a. temperature of the liquid b. volume of space above the liquid c. surface area of the liquid d. volume of the liquid QUESTION 9 Equal numbers of moles of \( \mathrm{He}(\mathrm{g}) \). Ar(g), and \( \mathrm{Ne}(\mathrm{g}) \) are placed in a glass vessel at room temperature. If the vessel has a pinhole-sized leak, which of the following will be true regarding the relative values of the partial pressure of the gases renfaining in the vessel after some of the gas mixture has effused? a. \( P_{\mathrm{Ne}_{e}}<\mathrm{P}_{\mathrm{Ar}}<\mathrm{P}_{\mathrm{He}} \) b. \( P_{H e}=P_{A t}=P_{\text {Ne }} \) c. \( P_{H e}