Home /
Expert Answers /
Chemistry /
a-240-0-mathrm-ml-buffer-solution-is-0-230-mathrm-m-in-acetic-acid-and-0-230-m-pa272
(Solved): A \( 240.0 \mathrm{~mL} \) buffer solution is \( 0.230 \mathrm{M} \) in acetic acid and \( 0.230 \m ...
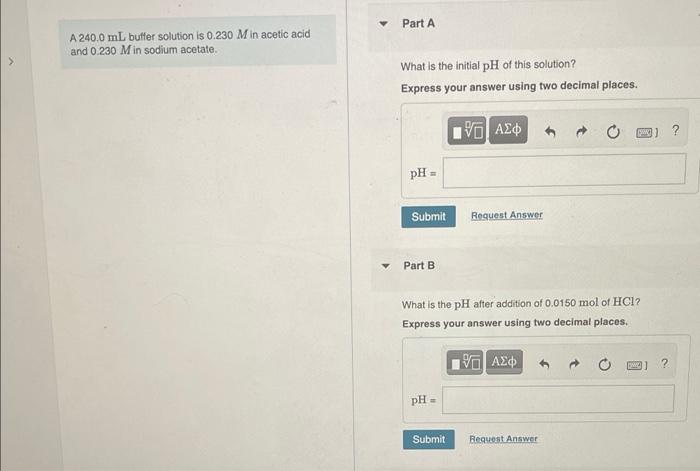

A \( 240.0 \mathrm{~mL} \) buffer solution is \( 0.230 \mathrm{M} \) in acetic acid and \( 0.230 \mathrm{M} \) in sodium acetate. What is the initial \( \mathrm{pH} \) of this solution? Express your answer using two decimal places. What is the \( \mathrm{pH} \) after addition of \( 0.0150 \mathrm{~mol} \) of \( \mathrm{HCl} \) ? Express your answer using two decimal places.
What is the \( \mathrm{pH} \) after addition of \( 0.0150 \mathrm{~mol} \) of \( \mathrm{NaOH} \) ? Express your answer using two decimal places.
Expert Answer
A) Ethanoic acid is a weak acid and dissociates: CH3COOH(aq)?CH3COO?(aq)+H+(aq) For which: Ka=[CH3COO?(aq)][H+(aq)]/ [CH3COOH(aq)]=1.8×10?5mol/l at 25?C Rearranging gives: [H+(aq)]=Ka×[CH3COOH(aq)]/[CH3COO?(aq)] Assume that equilibrium concentrations