Home /
Expert Answers /
Chemistry /
a-63-9-g-sample-of-benzene-c-6-h-6-molar-mass-78-11-g-m-ol-is-at-14-0-deg-c-if-25-00-kj-pa522
(Solved): A 63.9 g sample of benzene, C_(6)H_(6) (molar mass =78.11(g)/(m)ol ), is at 14.0\deg C. If 25.00 kJ ...
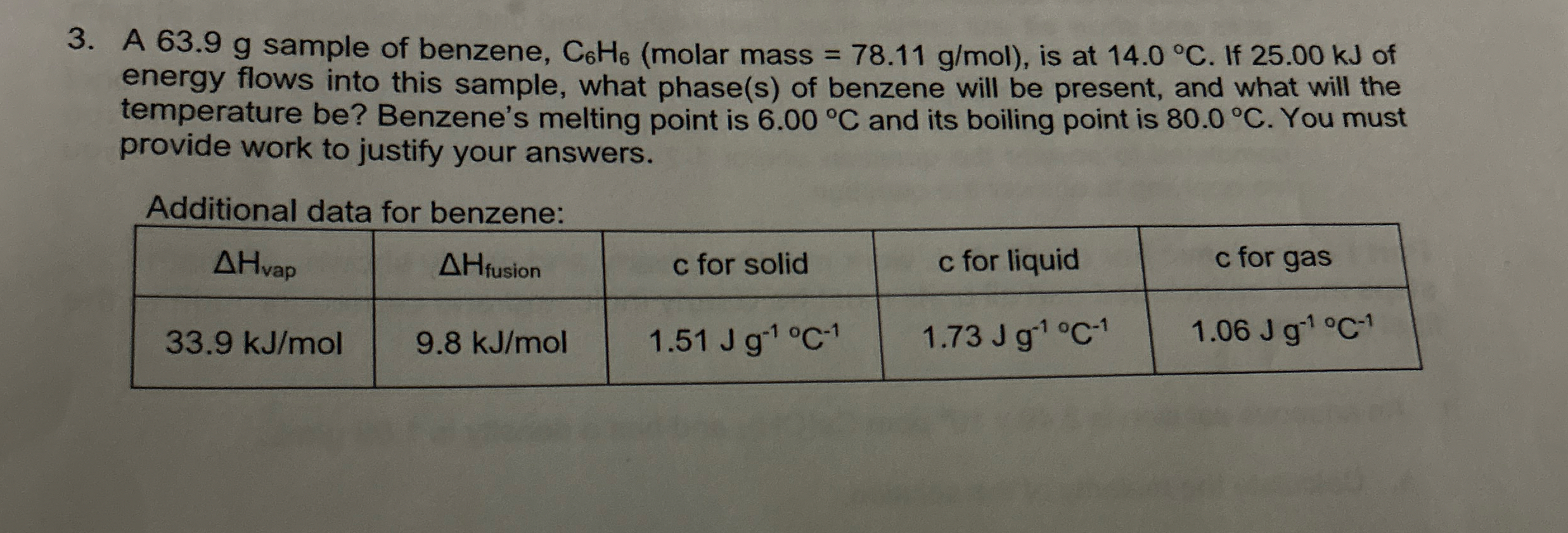
A 63.9 g sample of benzene,
C_(6)H_(6)(molar mass
=78.11(g)/(m)ol), is at
14.0\deg C. If 25.00 kJ of energy flows into this sample, what phase(s) of benzene will be present, and what will the temperature be? Benzene's melting point is
6.00\deg Cand its boiling point is
80.0\deg C. You must provide work to justify your answers. Additional data for benzene: \table[[
\Delta H_(vap ),
\Delta H_(fusion ),
cfor solid,
cfor liquid,
cfor gas],[
33.9k(J)/(m)ol,
9.8k(J)/(m)ol,
1.51Jg^(-1)C^(-1),
1.73Jg^(-1)\deg C^(-1),
1.06Jg^(-1)C^(-1)