Home /
Expert Answers /
Chemistry /
a-certain-chemical-reaction-releases-35-3-mathrm-kj-mathrm-g-of-heat-for-each-gram-of-pa223
(Solved): A certain chemical reaction releases \( 35.3 \mathrm{~kJ} / \mathrm{g} \) of heat for each gram of ...

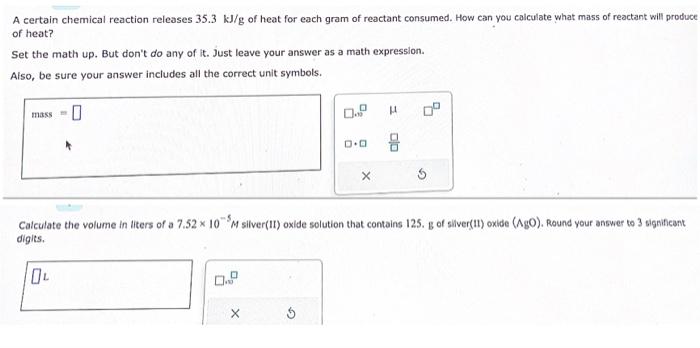
A certain chemical reaction releases \( 35.3 \mathrm{~kJ} / \mathrm{g} \) of heat for each gram of reactant consumed. How can you calculate what mass of reactant will produce of heat? Set the math up. But don't do any of it. Just leave your answer as a math expression. Also, be sure your answer includes all the correct unit symbols. Calculate the volume in liters of a \( 7.52 \times 10^{-5} \mathrm{M} \) silver(II) oxide solution that contains 125 . \( \mathrm{B} \) of sliver(II) oxide (A.B)). Round your answer to 3 significant digits,
A certain chemical reaction releases \( 35.3 \mathrm{~kJ} / \mathrm{g} \) of heat for each gram of reactant consumed. How can you calculate what mass of reactant will produce of heat? Set the math up. But don't do any of it. Just leave your answer as a math expression. Also, be sure your answer includes all the correct unit symbols. Calculate the volume in liters of a \( 7.52 \times 10^{-5} \mathrm{M} \) silver(II) oxide solution that contains 125. \( \mathrm{B} \) of silver(II) oxide (AgO). Round your answer to 3 significant digits,