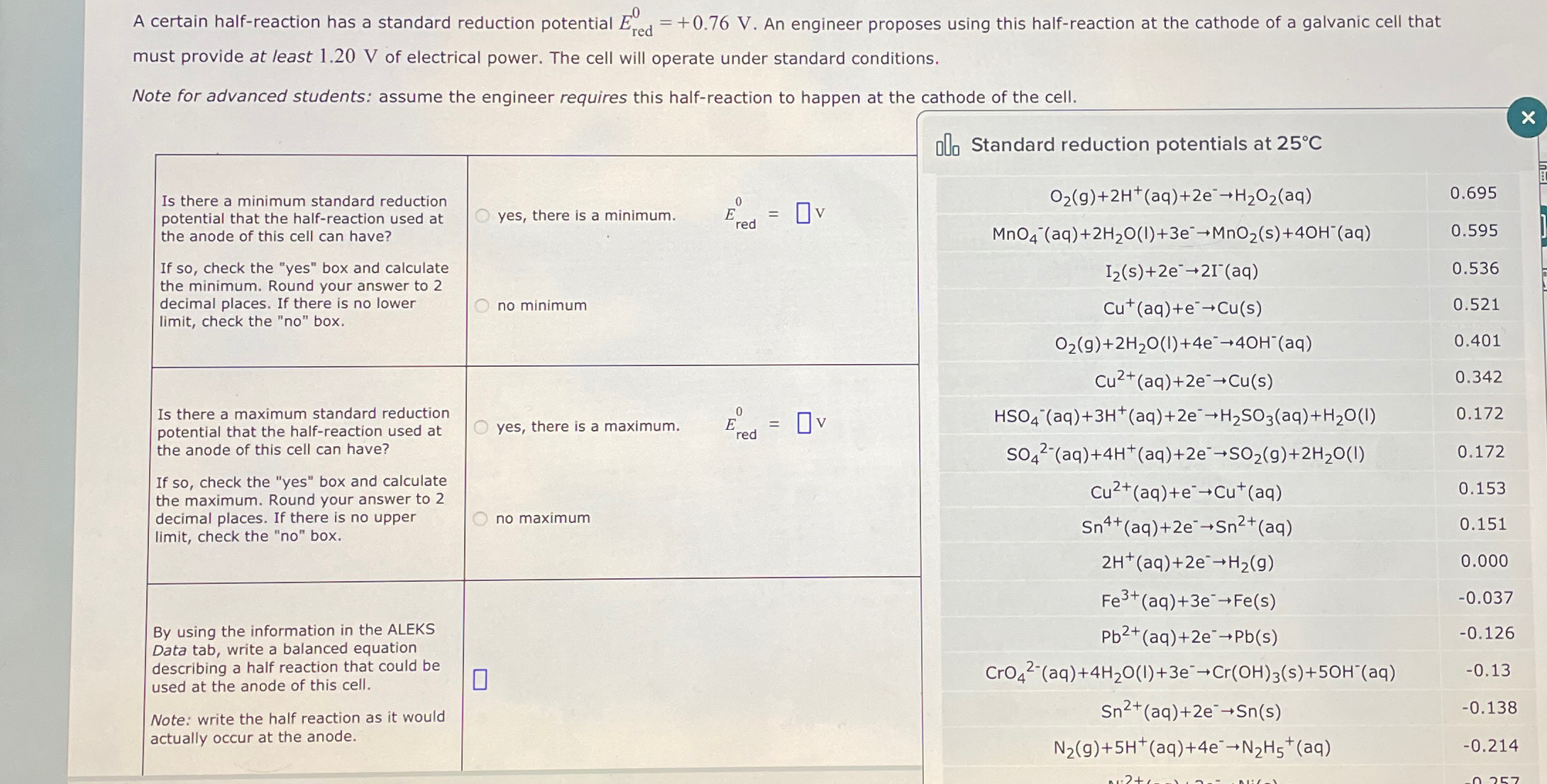
(Solved): A certain half-reaction has a standard reduction potential E_(red)^(0)=+0.76V. An engineer proposes ...
A certain half-reaction has a standard reduction potential
E_(red)^(0)=+0.76V. An engineer proposes using this half-reaction at the cathode of a galvanic cell that must provide at least
1.20Vof electrical power. The cell will operate under standard conditions. Note for advanced students: assume the engineer requires this half-reaction to happen at the cathode of the cell. \table[[Is there a minimum standard reduction,yes, there is a minimum.],[potential that the half-reaction used at,],[the anode of this cell can have?,],[If so, check the "yes" box and calculate,],[the minimum. Round your answer to 2,],[decimal places. If there is no lower,],[limit, check the "no" box.,no minimum]] ofo Standard reduction potentials at
25\deg C\table[[
O_(2)(g)+2H^(+)(aq)+2e^(-)->H_(2)O_(2)(aq),0.695],[
MnO_(4)^(-)(aq)+2H_(2)O(l)+3e^(-)->MnO_(2)(s)+4OH^(-)(aq),0.595],[
I_(2)(s)+2e^(-)->2I^(-)(aq),0.536],[
Cu^(+)(aq)+e^(-)->Cu(s),0.521],[
O_(2)(g)+2H_(2)O(l)+4e^(-)->4OH^(-)(aq),0.401],[
Cu^(2+)(aq)+2e^(-)->Cu(s),0.342],[
HSO_(4)^(-)(aq)+3H^(+)(aq)+2e^(-)->H_(2)SO_(3)(aq)+H_(2)O(I),0.172],[
SO_(4)^(2-)(aq)+4H^(+)(aq)+2e^(-)->SO_(2)(g)+2H_(2)O(I),0.172],[
Cu^(2+)(aq)+e^(-)->Cu^(+)(aq),0.153],[
Sn^(4+)(aq)+2e^(-)->Sn^(2+)(aq),0.151],[
2H^(+)(aq)+2e^(-)->H_(2)(g),0.000],[
Fe^(3+)(aq)+3e^(-)->Fe(s),-0.037],[
Pb^(2+)(aq)+2e^(-)->Pb^(2)(s),-0.126],[
CrO_(4)^(2-)(aq)+4H_(2)O(l)+3e^(-)->Cr(OH)_(3)(s)+5OH^(-)(aq),-0.13],[
Sn^(2+)(aq)+2e^(-)->Sn(s),-0.138],[
N_(2)(g)+5H^(+)(aq)+4e^(-)->N_(2)H_(5)^(+)(aq),-0.214]]