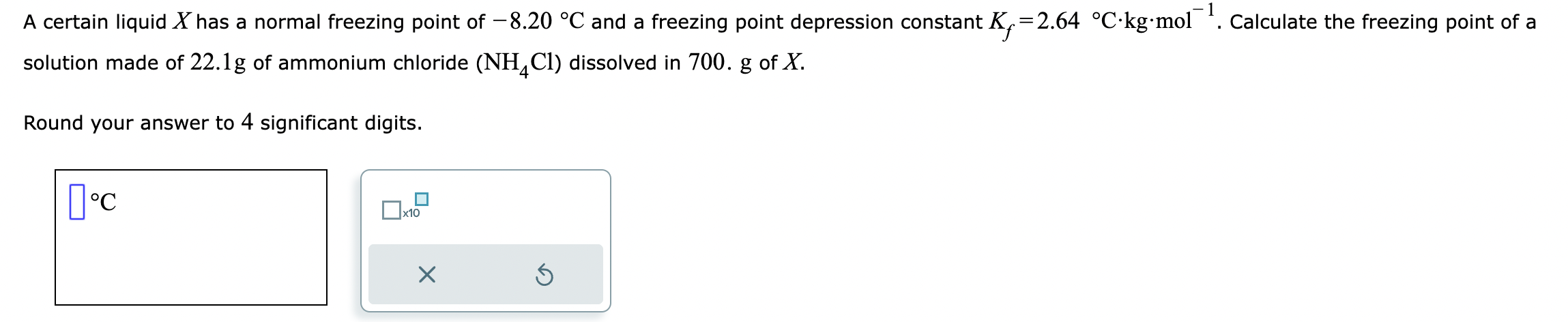Home /
Expert Answers /
Chemistry /
a-certain-liquid-x-has-a-normal-freezing-point-of-8-20-circ-mathrm-c-and-a-freez-pa718
(Solved): A certain liquid \( X \) has a normal freezing point of \( -8.20^{\circ} \mathrm{C} \) and a freez ...
A certain liquid \( X \) has a normal freezing point of \( -8.20^{\circ} \mathrm{C} \) and a freezing point depression constant \( K_{f}=2.64{ }^{\circ} \mathrm{C} \cdot \mathrm{kg} \cdot \) mol \( { }^{-1} \). Calculate the freezing point of a solution made of \( 22.1 \mathrm{~g} \) of ammonium chloride \( \left(\mathrm{NH}_{4} \mathrm{Cl}\right) \) dissolved in 700 . \( \mathrm{g} \) of \( X \). Round your answer to 4 significant digits.