Home /
Expert Answers /
Chemistry /
a-chemist-carefully-measures-the-amount-of-heat-needed-to-raise-the-temperature-of-a-1-14kg-sampl-pa528
(Solved): A chemist carefully measures the amount of heat needed to raise the temperature of a 1.14kg sampl ...
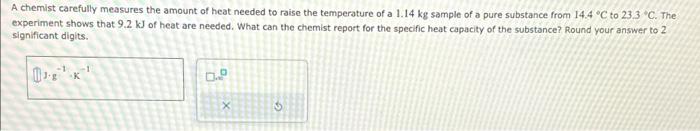
A chemist carefully measures the amount of heat needed to raise the temperature of a sample of a pure substance from ° to " . The experiment shows that of heat are needed. What can the chemist report for the specific heat capacity of the substance? Round your answer to 2 . significant digits.
Expert Answer
Given data,mass = 1.14 kgenergy = 9.2 kJinitial temperature = 14.4 oCinitial temperature = 23.3 oCwe have to calculate specific heat capacity=?