Home /
Expert Answers /
Chemistry /
a-chemistry-graduate-student-is-given-450-ml-of-a-1-60m-benzouc-acid-hc6h5co2-sclution-pa314
(Solved): A chemistry graduate student is given 450 . mL of a 1.60M benzouc acid (HC6H5CO2) sclution ...
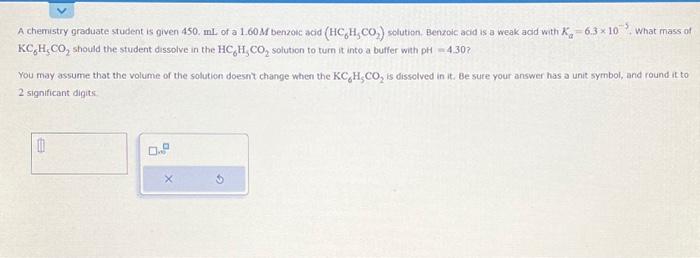
A chemistry graduate student is given 450 . mL of a benzouc acid sclution. Benzoic acid is a weak acid with . What mass of should the student dissolve in the solution to turn it into a butfer with ? You may assume that the volume of the solution doesnt change when the is dissolved in it, Be sute your answer has a unit symbol, and round it to 2 significant digits
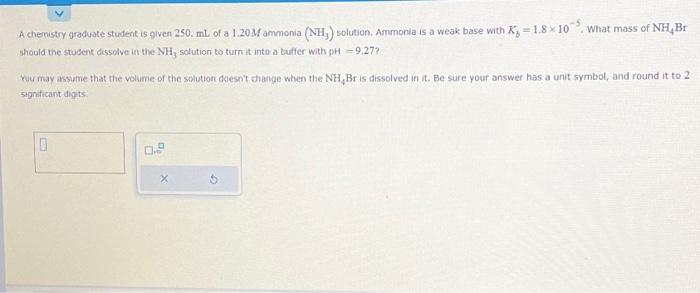
A chemistry graduate student is given of a ammonia solution. Ammonia is a weak base with . What mass of should the stubent dissolve in the solution to turn it into a bulfer with ? You may assume that the volume of the soluthon qhesn't thange when the is dissolved in it. Be sure your answer has a unit symbol, and round it to 2 significant dipts:
Expert Answer
Given that, Concentration of a ammonia = 1.60M (weak base)Volume of solution = 450mL =0.450L Moles = concentration × volume Moles of Benzoic acid= 1.60M × 0.450L = 0.72moles pH= 4.30 is the negative logarithm of the valu of Buffer solution: the solution of weak acid and it's salt or weak base and it's salt is ls known as buffer