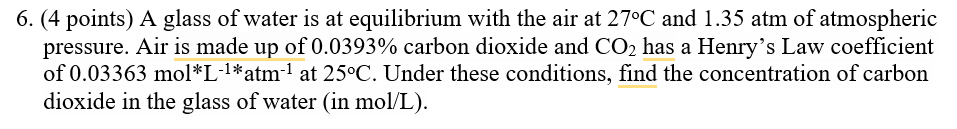Home /
Expert Answers /
Civil Engineering /
a-glass-of-water-is-at-equilibrium-with-the-air-at-27oc-and-1-35-atm-of-atmospheric-pressure-air-is-pa957
(Solved): A glass of water is at equilibrium with the air at 27oC and 1.35 atm of atmospheric pressure. Air is ...
A glass of water is at equilibrium with the air at 27oC and 1.35 atm of atmospheric pressure. Air is made up of 0.0393% carbon dioxide and CO2 has a Henry’s Law coefficient of 0.03363 mol*L-1*atm-1 at 25oC. Under these conditions, find the concentration of carbon dioxide in the glass of water (in mo(l)/(L)).