Home /
Expert Answers /
Chemistry /
a-here-is-a-proposed-sn-1-mechanism-h-5-br-39-s-n-in-this-mechanism-there-are-two-mistakes-one-pa546
(Solved): a. Here is a proposed Sn 1 Mechanism: H-5 :Br: 'S N In this mechanism there are two mistakes; one ...
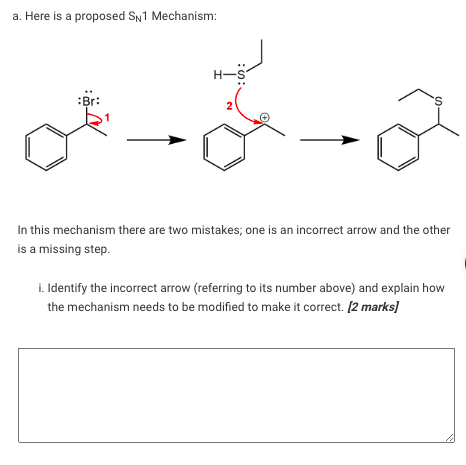
a. Here is a proposed Sn 1 Mechanism: H-5 :Br: 'S N In this mechanism there are two mistakes; one is an incorrect arrow and the other is a missing step 1. Identify the incorrect arrow (referring to its number above) and explain how the mechanism needs to be modified to make it correct. [2 marks]
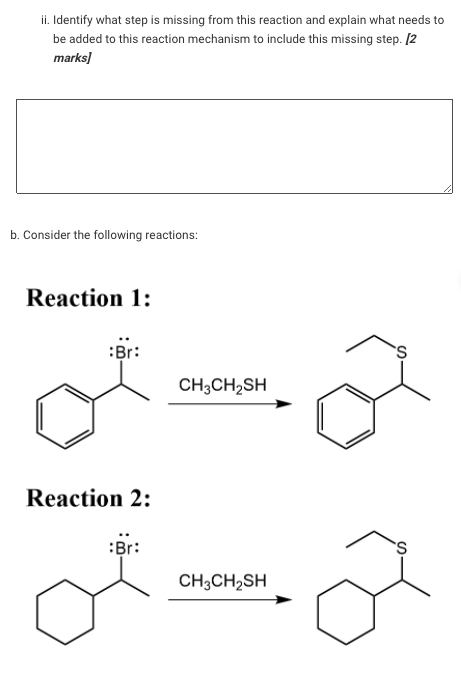
ii. Identify what step is missing from this reaction and explain what needs to be added to this reaction mechanism to include this missing step. 12 marks b. Consider the following reactions: Reaction 1: :Br: 'S CH3CH2SH Reaction 2: Br: CH3CH2SH
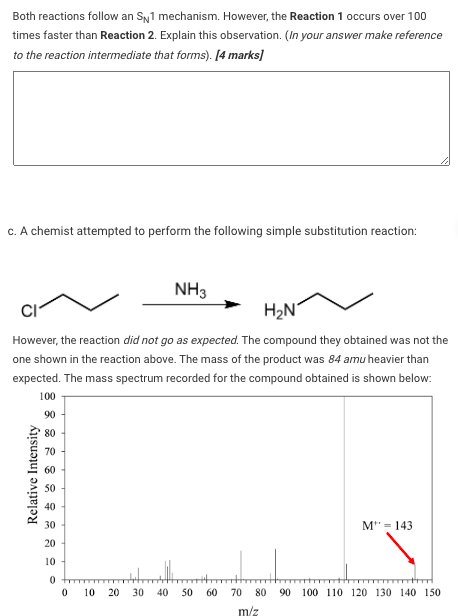
Both reactions follow an Sp1 mechanism. However, the Reaction 1 occurs over 100 times faster than Reaction 2. Explain this observation. (In your answer make reference to the reaction intermediate that forms). [4 marks] C. A chemist attempted to perform the following simple substitution reaction: NH3 H2N However, the reaction did not go as expected. The compound they obtained was not the one shown in the reaction above. The mass of the product was 84 amu heavier than expected. The mass spectrum recorded for the compound obtained is shown below: 100 90 80 70 60 Relative Intensity 50 40 30 M"=143 20 10 . 0 0 10 20 30 40 50 60 90 100 110 120 130 140 150 70 80 m/z
1. Identify the product obtained and draw it in the canvas below. [2 marks] Your current response is: ChemDoodle Click here to open the canvas ii. Explain how this product was able to form instead of the product shown in the reaction scheme above. [1 mark]
Expert Answer
You have asked more questions since at once we can answer only one question. Here I'm giving the answer to the first two questions. i.e. a and b In the first step: The given compound contains a bromine group at the benzylic position. Hence in the SN1
![1. Identify the product obtained and draw it in the canvas below. [2 marks]
Your current response is:
ChemDoodle
Click here t](https://media.cheggcdn.com/media/71e/71ef6b8c-dd87-41ab-a0f1-a26d33640930/phpAlcfHZ)