Home /
Expert Answers /
Chemistry /
a-method-for-producing-the-compound-mathrm-c-2-mathrm-h-6-mathrm-pa977
(Solved): A method for producing the compound \( \mathrm{C}_{2} \mathrm{H}_{6}(\mathrm{ ...
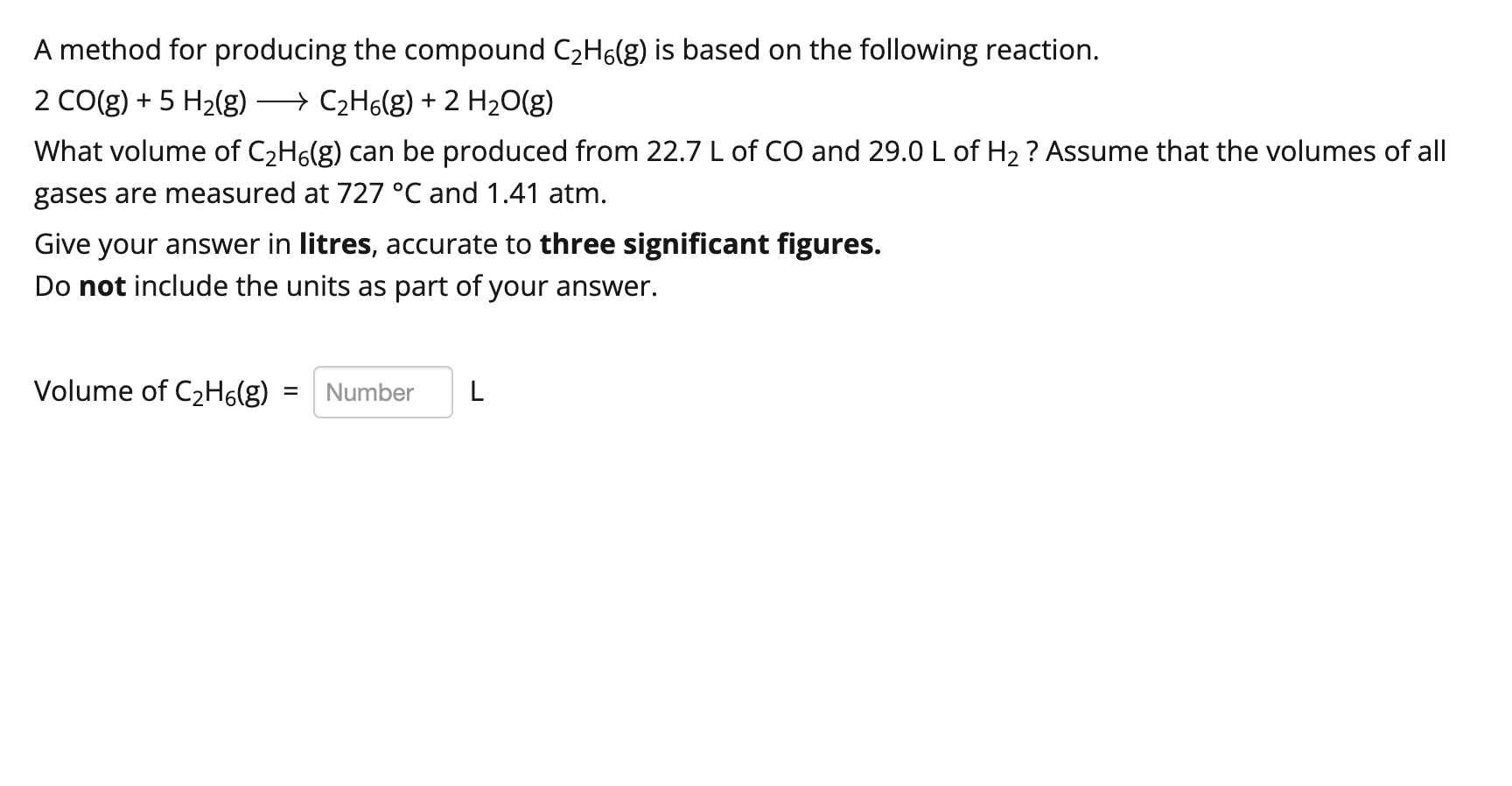
A method for producing the compound \( \mathrm{C}_{2} \mathrm{H}_{6}(\mathrm{~g}) \) is based on the following reaction. \[ 2 \mathrm{CO}(\mathrm{g})+5 \mathrm{H}_{2}(\mathrm{~g}) \longrightarrow \mathrm{C}_{2} \mathrm{H}_{6}(\mathrm{~g})+2 \mathrm{H}_{2} \mathrm{O}(\mathrm{g}) \] What volume of \( \mathrm{C}_{2} \mathrm{H}_{6}(\mathrm{~g}) \) can be produced from \( 22.7 \mathrm{~L} \) of \( \mathrm{CO} \) and \( 29.0 \mathrm{~L} \) of \( \mathrm{H}_{2} \) ? Assume that the volumes of all gases are measured at \( 727^{\circ} \mathrm{C} \) and \( 1.41 \) atm. Give your answer in litres, accurate to three significant figures. Do not include the units as part of your answer. Volume of \( \mathrm{C}_{2} \mathrm{H}_{6}(\mathrm{~g})= \)
Expert Answer
Solution: Concepts Used: 1.Limiting Reagent; Limiting reagents are those which exhausted as early in the reaction. Steps: Step:01:Write the given