Home /
Expert Answers /
Chemistry /
a-mixture-initially-contains-a-b-and-c-in-the-following-concentrations-a-0-700moll1-b-pa652
(Solved): A mixture initially contains A,B, and C in the following concentrations: [A]=0.700molL1,[B ...
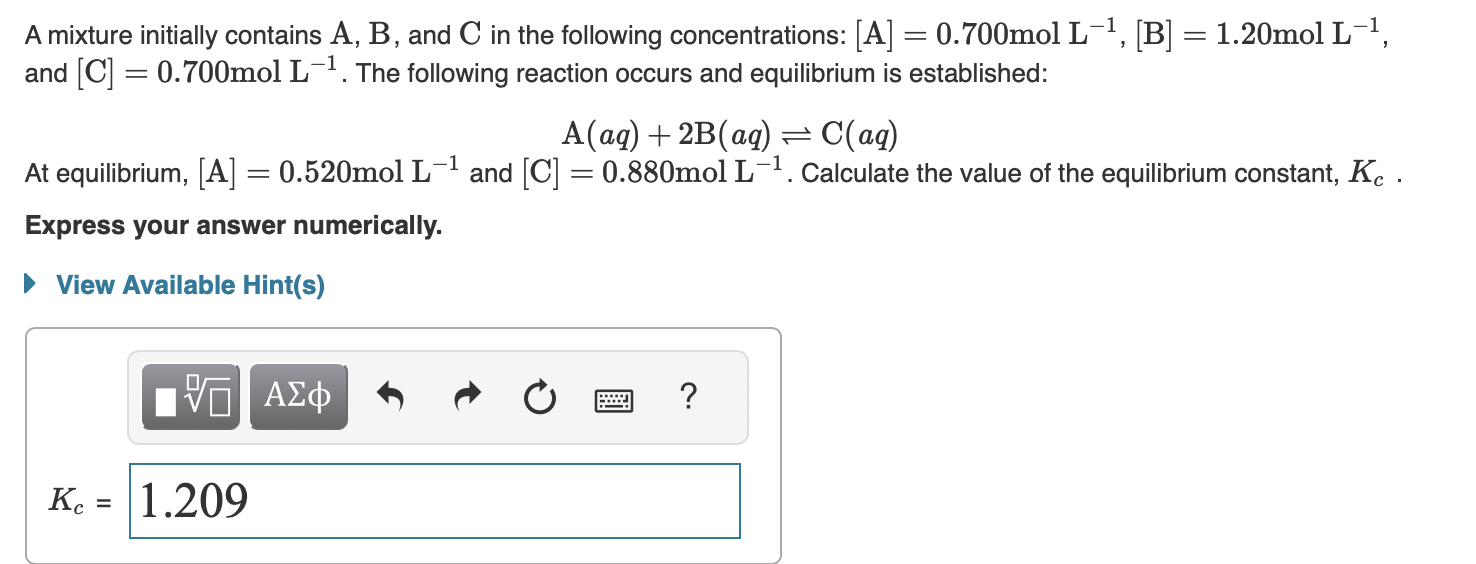
A mixture initially contains , and in the following concentrations: , and . The following reaction occurs and equilibrium is established: At equilibrium, and . Calculate the value of the equilibrium constant, . Express your answer numerically.
Expert Answer
The given reaction is as follows,Concentration Initial 0.700 1.20 ...