Home /
Expert Answers /
Other Math /
a-mixture-of-gas-contains-3-2-mathrm-kg-of-oxygen-2-2-mathrm-kg-of-carbon-dioxi-pa853
(Solved): A mixture of gas contains \( 3.2 \mathrm{~kg} \) of Oxygen, \( 2.2 \mathrm{~kg} \) of Carbon Dioxi ...
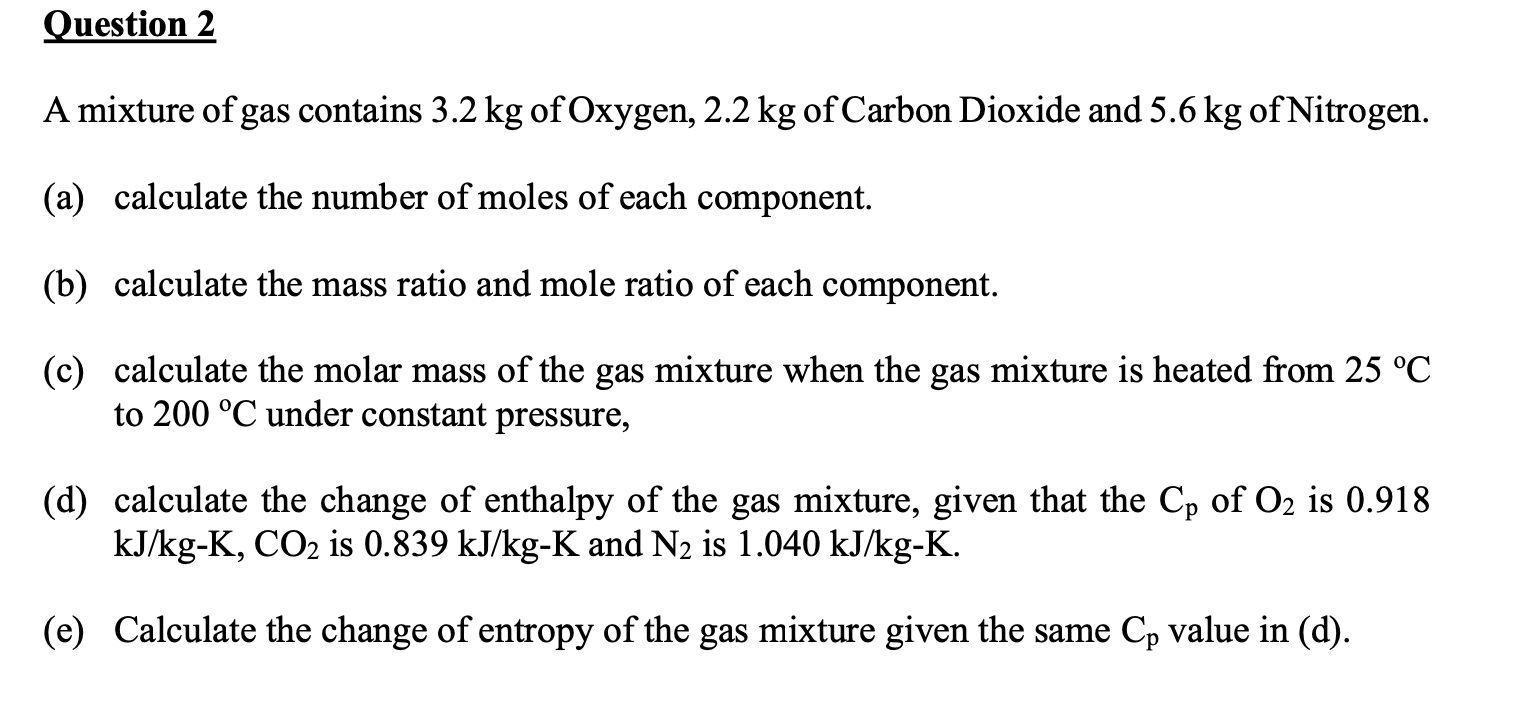
A mixture of gas contains \( 3.2 \mathrm{~kg} \) of Oxygen, \( 2.2 \mathrm{~kg} \) of Carbon Dioxide and \( 5.6 \mathrm{~kg} \) of Nitrogen. (a) calculate the number of moles of each component. (b) calculate the mass ratio and mole ratio of each component. (c) calculate the molar mass of the gas mixture when the gas mixture is heated from \( 25^{\circ} \mathrm{C} \) to \( 200^{\circ} \mathrm{C} \) under constant pressure, (d) calculate the change of enthalpy of the gas mixture, given that the \( \mathrm{C}_{\mathrm{p}} \) of \( \mathrm{O}_{2} \) is \( 0.918 \) \( \mathrm{kJ} / \mathrm{kg}-\mathrm{K}, \mathrm{CO}_{2} \) is \( 0.839 \mathrm{~kJ} / \mathrm{kg}-\mathrm{K} \) and \( \mathrm{N}_{2} \) is \( 1.040 \mathrm{~kJ} / \mathrm{kg}-\mathrm{K} \). (e) Calculate the change of entropy of the gas mixture given the same \( \mathrm{C}_{\mathrm{p}} \) value in (d).