Home /
Expert Answers /
Chemistry /
a-rfading-a-graph-a-graph-is-a-visual-display-of-information-grophs-show-relationahips-betwees-n-pa256
(Solved): A. RFADING A GRAPH: A graph is a visual display of information. Grophs show relationahips betwees n ...


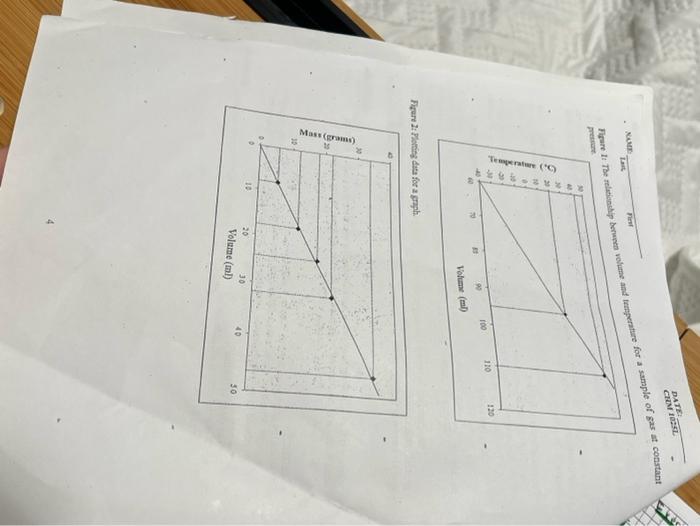
A. RFADING A GRAPH: A graph is a visual display of information. Grophs show relationahips betwees namerical) quantities in picture form in order to make the information clearer and eagier to understand. For example, the graph in Figure 1 shows the relationatip between the teroperature and the volume of a sample of gas at constant ptessure. To read and underntand the information in Figare 1, we must study the deseription of the vertical axis (ordinate) and borizontal axis (abseisa). The vertical axis is babelod temperature (in degrees 'C). The ligher up the vertical axis one goes in Figure 1 the grester the temperature. The horizontal axis is labeled volume (in milhiliters). The further to the right on the berizontal wais one goes in Figurel, the greater is the voluans. The solid lise is this graph stows ws the volume of this sample of gas at various temperatures. Suppose we wish to determine the volume of the gas at \( 20^{\circ} \mathrm{C} \). We wowld start at \( 20^{\circ} \mathrm{C} \) on the vertical axis and move to the right until wo reached the line (polat A). The volume at this temperature is found directly below poins \( \mathrm{A} \) on the botizontal acis. The volume is about \( 95 \mathrm{ml} \). Suppose we wist to determine the tempenause of the gas when its volume in \( 110 \mathrm{ml} \), we could start at \( 110 \mathrm{ml} \), on the borizontal axis and move up until we reach the line (point B). The temperafure at this volume is found directly to the left of point 9 on the vertical axis. The temperature is abous \( 43^{\circ} \mathrm{C} \) Using Figure 1, find the following information: a. the volume at \( 40^{\circ} \mathrm{C} \). b. the volume at \( 20^{\circ} \mathrm{C} \) c. the temperature when the volume \( 90 \mathrm{ml} \) d. the temperature when the volurae is \( 100 \mathrm{ml} \) l B. PI,CTTUIING A GRARH: Construct a graph by using the following data for tho volume and mass of an unknowa solid. You mxy find it useful to make a graph of numerieal information that you bave obtained in the laboratory. For exair was:
It if acceipary as chaspe cach reating ta a poitt on the graph. Readeng 1 is \( 8.02 \) mul and located. Tbea we move directly up frem that poief atil we reach \( 5.71 \) on the vertical axis. We: a Cenitruct a grapb by uting the following set of data for the volime and mas of an unkikontl sist Codived a groph by nuing the following data ay tha tolunte a sanple of gas occupies at vatious ?otssuret.
The line shown in Fig. 2 was constructed from labotatory dota. Tbe line repecients the average of five readings and should be moce aceurate than the individual readings. The line may be used to determine the mass of the substance if the volume is knows ot vice versa. Since the graph in Fig 2 shows the relationabip between mass asd volume for a substance, and since density is a relationship between mass and volume, we can ose the groph to detcranino the density of tha substanco. The procedure is as follows: a. Pick two widely separated points on the line: For example: \( 10.0 \mathrm{ml}, 7.1 \) g and \( 40.0 \mathrm{ml}, 28.4 \mathrm{~g} \) b. The density is given by the change in mass divided by the change in volume between these two points Density \( =\frac{\text { Changs in Mass }}{\text { Chatage tn Volame }}=\frac{29.4 \mathrm{~s}-7.1 \mathrm{~g}}{40.0 \mathrm{ml}-10.0 \mathrm{ml}}=\frac{213 \mathrm{~g}}{30.0 \mathrm{ml}}=\frac{0.710 \mathrm{~g}}{\mathrm{ml}} \) The procedure by which a change in the \( y \) axis is divided by a change in the \( x \) axis is a geveral method applicable to any straight-line graph. The term calculated \( (0.710 \mathrm{gjm}) \) in this case) is known as the slope of the line. Any straight-line graph can be expressed by the equation: \[ y=m x+b \] where \( y \) is the value along the \( y \) axis at a point on the line, \( x \) is the value along the \( x \) sais at the same point, \( m \) is the slope of the line, and \( b \) is the value of \( y \) where the line crosses the \( y \) axis (b \( =0 \) in fig \( A .1 .2 \) ). For Fig. A. \( 12, y=0.710 x \). Exercise 4: By using the graphs you drew in Exercise 1 and 2 determine: a. The density of the substance for exercise 1 and exercise 2. Submit the grophs properly labeled to your instructor for evaluation after you have calculated their detsity from the b. The slope of the line c. Write the general equation for the line in the form \( y=m x+b \) with the appropriste values for \( \mathrm{m} \) and \( \mathrm{b} \).
- Nutt nat for 2 sample of gax at constant Bigere 1t Plowisz dits for a groph.
Expert Answer
I have solved the question as per the chegg guidelines.. your image is little blurred..so please if by mistake i input wrong answer please