Home /
Expert Answers /
Chemistry /
a-rock-contains-0-538-mg-206-pb-for-every-1-000-mg-238-u-present-assuming-that-no-lead-was-orig-pa163
(Solved): A rock contains 0.538 mg ^(206)Pb for every 1.000 mg ^(238)U present. Assuming that no lead was orig ...
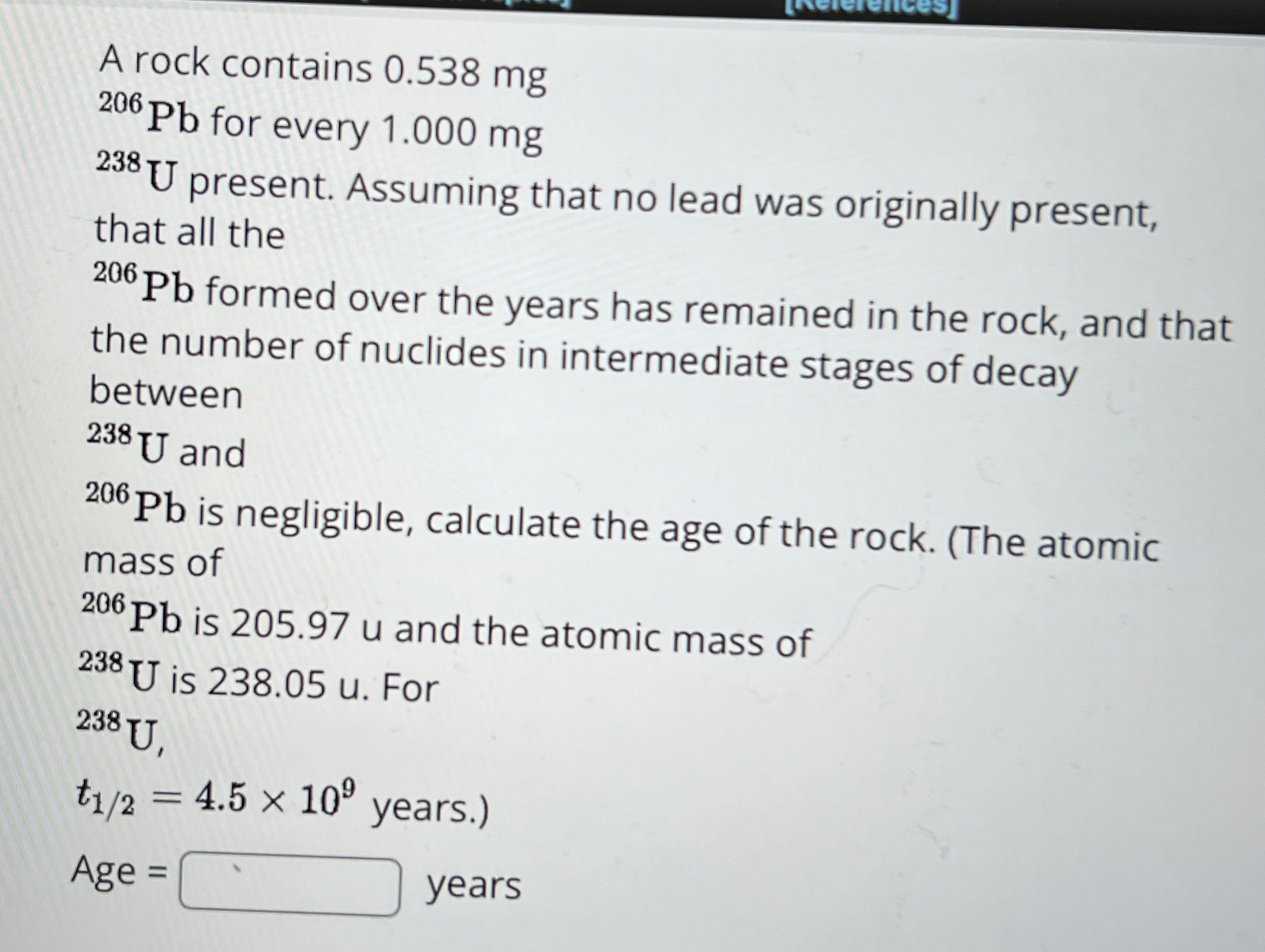
A rock contains 0.538 mg
^(206)Pbfor every 1.000 mg
^(238)Upresent. Assuming that no lead was originally present, that all the
^(206)Pbformed over the years has remained in the rock, and that the number of nuclides in intermediate stages of decay between
^(238)Uand
^(206)Pbis negligible, calculate the age of the rock. (The atomic mass of
^(206)Pbis 205.97 u and the atomic mass of
^(238)Uis 238.05 . For
^(238)U,
t_((1)/(2))=4.5\times 10^(9)years.) Age
=
◻years