Home /
Expert Answers /
Chemistry /
a-student-gets-the-following-data-for-the-heat-of-neutralization-trial-using-1-0mnaoh-and-1-0mhcl-pa975
(Solved): A student gets the following data for the Heat of Neutralization Trial using 1.0MNaOH and 1.0MHCl ...
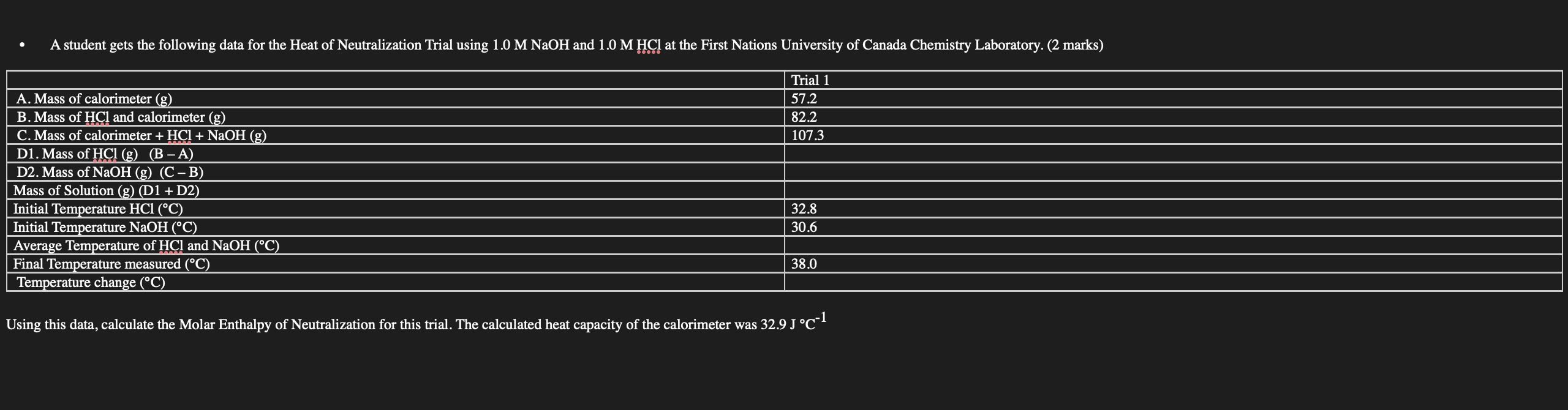
A student gets the following data for the Heat of Neutralization Trial using and at the First Nations University of Canada Chemistry Laboratory. (2 marks) Using this data, calculate the Molar Enthalpy of Neutralization for this trial. The calculated heat capacity of the calorimeter was
Expert Answer
The total heat released by the reaction is the sum of the heat released by the acid and the heat absorbed by the base:qtotal =q(HCl)+q(NaOH)qtotal = m