Home /
Expert Answers /
Chemistry /
answer-both-plz-charge10-an-atom-with-53-protons-and-54-electrons-has-a-formula-of-xe-xe-perio-pa328
(Solved): answer both plz Charge10. An atom with 53 protons and 54 electrons has a formula of Xe Xe+ Perio ...
answer both plz



Charge10. An atom with 53 protons and 54 electrons has a formula of
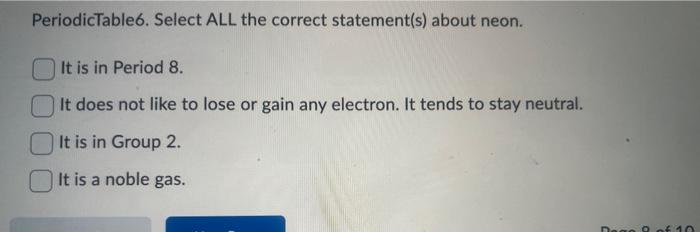
PeriodicTable6. Select ALL the correct statement(s) about neon. It is in Period 8. It does not like to lose or gain any electron. It tends to stay neutral. It is in Group 2. It is a noble gas.
Expert Answer
Charge10. An atom with 53 protons and 54 electrons has a formula of option D i.e., IA? .Atomic number = number of protonsdata