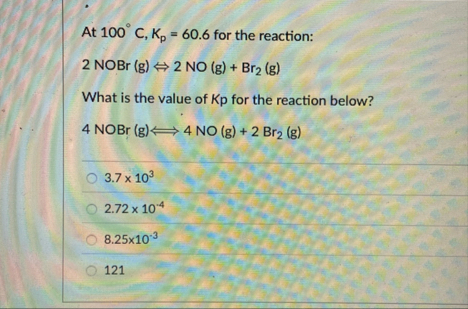Home /
Expert Answers /
Chemistry /
at-100-deg-c-k-p-60-6-for-the-reaction-2nobr-g-lt-gt-2no-g-br-2-g-what-is-the-value-of-kp-pa691
(Solved): At 100\deg C,K_(p)=60.6 for the reaction: 2NOBr(g)<=>2NO(g) Br_(2)(g) What is the value of Kp ...
At
100\deg C,K_(p)=60.6for the reaction:
2NOBr(g)<=>2NO(g) Br_(2)(g)What is the value of
Kpfor the reaction below?
4NOBr(g)Longleftrightarrow4NO(g) 2Br_(2)(g)
3.7\times 10^(3)
2.72\times 10^(-4)
8.25\times 10^(-3)121