Home /
Expert Answers /
Chemistry /
at-a-certain-temperature-the-vapor-pressure-of-pure-acetyl-bramide-left-mathrm-ch-3-mathrm-pa786
(Solved): At a certain temperature the vapor pressure of pure acetyl bramide \( \left(\mathrm{CH}_{3} \mathrm ...
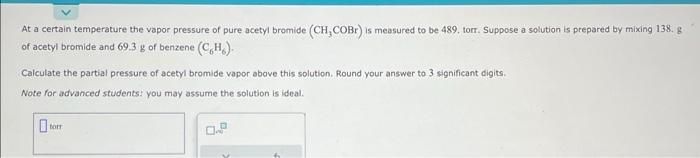
At a certain temperature the vapor pressure of pure acetyl bramide \( \left(\mathrm{CH}_{3} \mathrm{COBr}\right) \) is measured to be 489 , torr. Suppose a solution is prepared by mixing \( 138.8 \) of acetyl bromide and \( 69.3 \mathrm{~g} \) of benzene \( \left(\mathrm{C}_{6} \mathrm{H}_{6}\right) \). Calculate the partial pressure of acetyl bromide vapor above this solution. Round your answer to 3 significant digits. Note for advanced students: you may assume the solution is ideal.
Expert Answer
moles of acetyl bromide in mixture = mass / molar mass = 138 g / 123 g/mol = 1.12195 moles of ben