Home /
Expert Answers /
Chemistry /
at-a-certain-temperature-this-reaction-follows-second-order-kinetics-with-a-rate-constant-of-2-90m-pa619
(Solved): At a certain temperature this reaction follows second-order kinetics with a rate constant of 2.90M^( ...
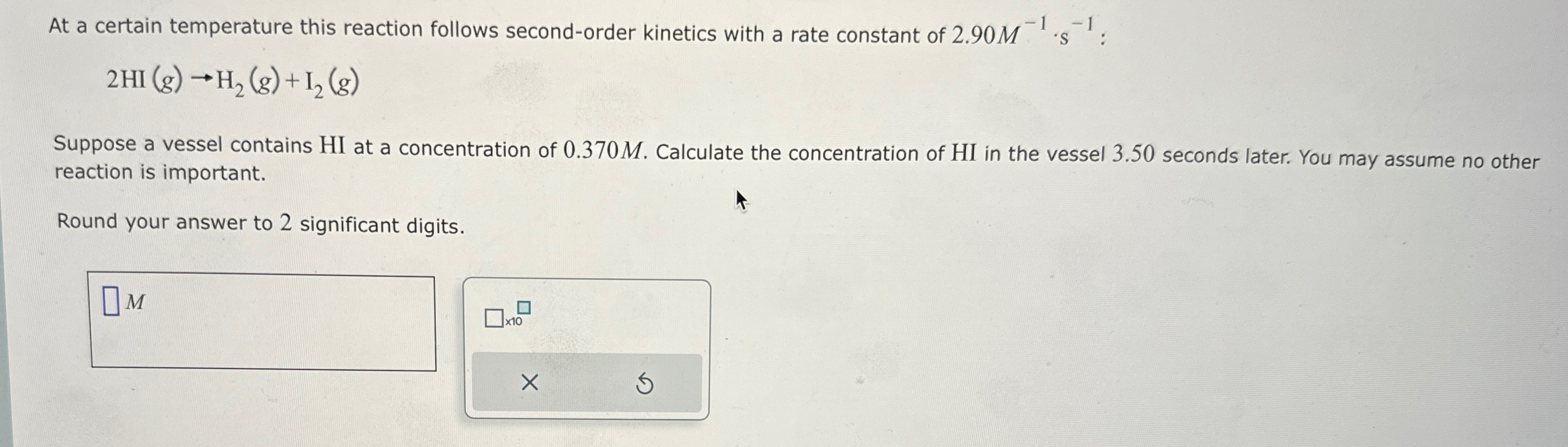
At a certain temperature this reaction follows second-order kinetics with a rate constant of
2.90M^(-1)*s^(-1):
2HI(g)->H_(2)(g)+I_(2)(g)Suppose a vessel contains
HIat a concentration of
0.370M. Calculate the concentration of
HIin the vessel 3.50 seconds later. You may assume no other reaction is important. Round your answer to 2 significant digits.