Home /
Expert Answers /
Chemistry /
b-if-you-start-with-0-045ml2-at-this-temperature-how-much-will-remain-after-5-25-s-assuming-t-pa890
(Solved): (b) If you start with 0,045Ml2 at this temperature, how much will remain after 5.25 s assuming t ...
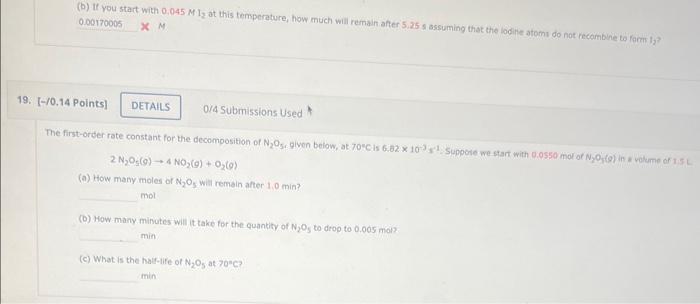
(b) If you start with at this temperature, how much will remain after s assuming that the loolite atoms do not recainbine ta farm la? X M. Submissions Used (a) How many moles of will remain after 1,0 min? mol. (b) How many minutes will it take for the quantity of to drop to mol? min (c) What is the half-life of at ? min
Expert Answer
The given reaction is 2NA2OA5?4NOA2+OA2for a first order reaction, the equation is [N2O5]t=[N2O5]oe??tSince Volume is constant, the