Home /
Expert Answers /
Chemistry /
balance-and-indicate-what-type-or-types-of-reactions-each-of-the-following-represents-precipita-pa589
Expert Answer
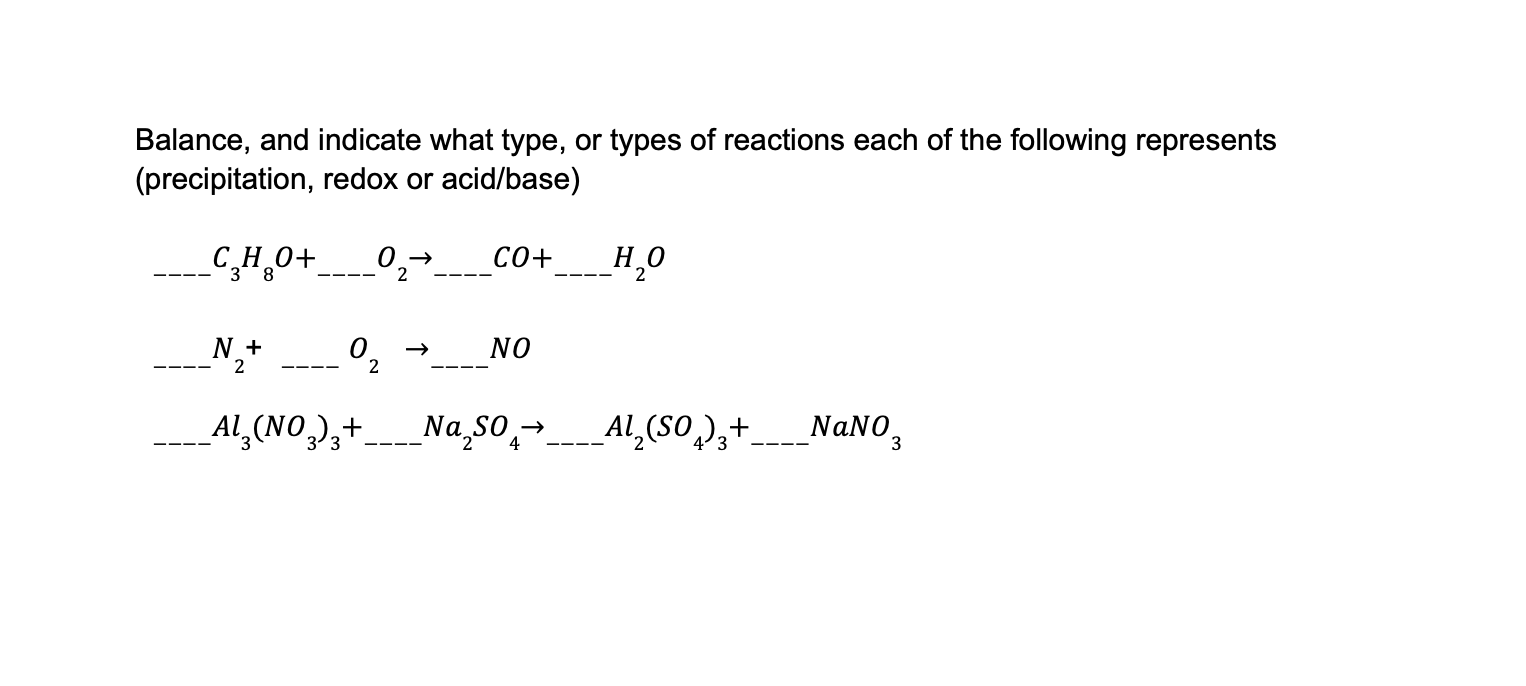
a. This reaction represents a combustion reaction, which is a type of redox reaction. In combustion reactions, a substance reacts with oxygen to produce carbon dioxide and water. In this case, C?H8O (ethanol) reacts with O? (oxygen) to produce CO (carbon monoxide) and H?O (water).In this reaction, ethanol (a type of alcohol) combines with oxygen to produce carbon monoxide and water. This reaction is called combustion, which is a process that occurs when a substance reacts with oxygen and releases heat and light.