Home /
Expert Answers /
Chemistry /
balanced-equation-cucl22h2o-2al-3cu-2alcl3-6h2o-answer-the-following-questions-bef-pa582
(Solved): Balanced equation: CuCl22H2O+2Al=3Cu+2AlCl3+6H2O Answer the following questions BEF ...
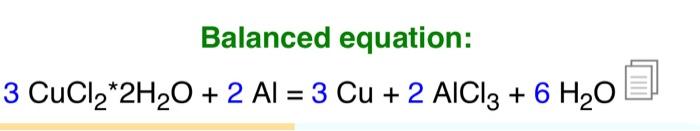
Balanced equation:
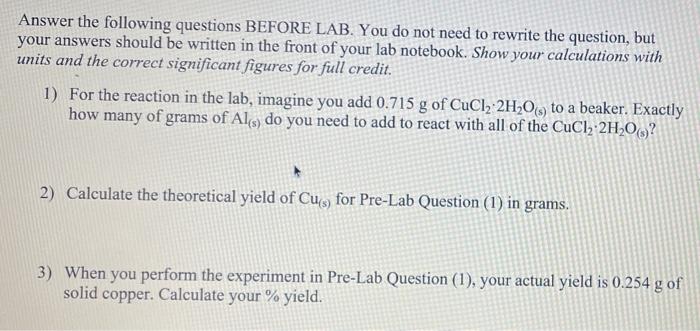
Answer the following questions BEFORE LAB. You do not need to rewrite the question, but your answers should be written in the front of your lab notebook. Show your calculations with units and the correct significant figures for full credit. 1) For the reaction in the lab, imagine you add of to a beaker. Exactly how many of grams of do you need to add to react with all of the ? 2) Calculate the theoretical yield of for Pre-Lab Question (1) in grams. 3) When you perform the experiment in Pre-Lab Question (1), your actual yield is of solid copper. Calculate your \% yield.