Home /
Expert Answers /
Chemistry /
bonus-cobalt-nitrate-actually-used-for-ms-actual-ms-actual-ws5-how-would-you-go-about-making-a-pa355
(Solved): Bonus: Cobalt nitrate actually used for MS Actual MS Actual WS5.) How would you go about making a ...
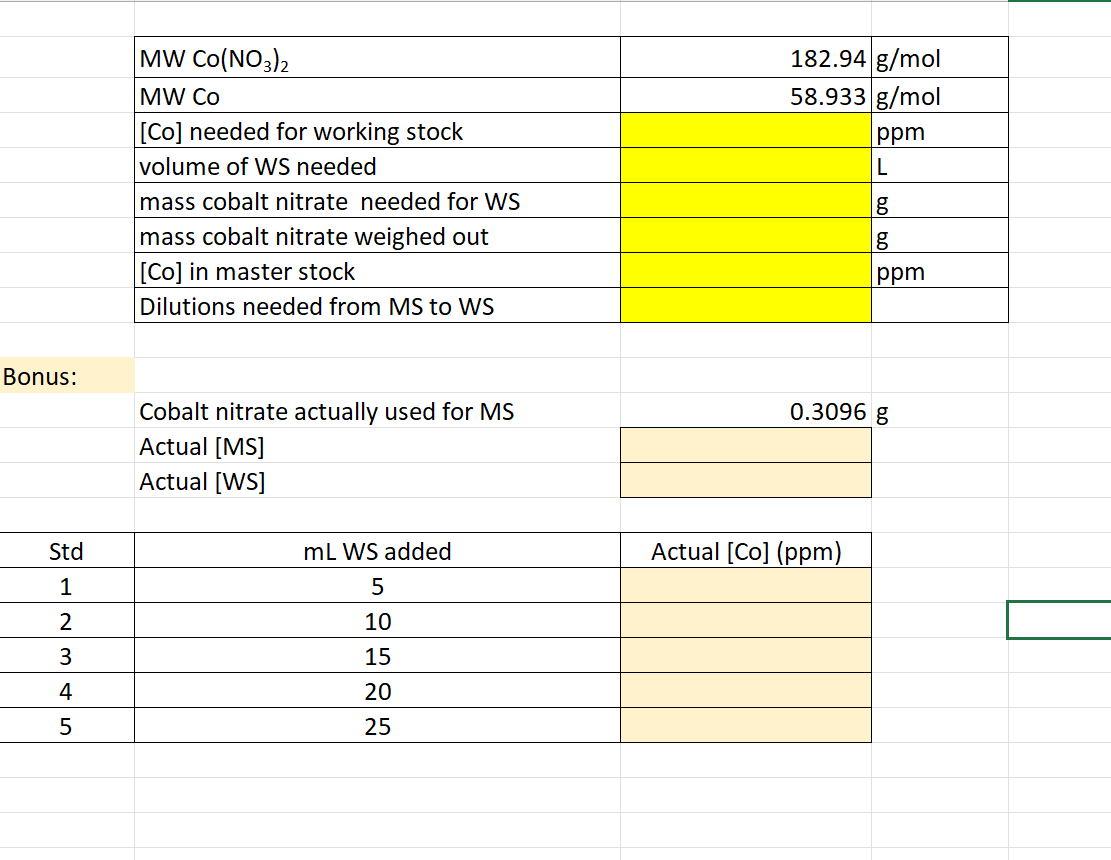
Bonus: Cobalt nitrate actually used for MS Actual
MSActual
WS5.) How would you go about making a set of standards ranging from
1.0-5.0ppmfor a test measuring cobalt
(Co,58.933(g)/(m)ol)in natural water samples using cobalt nitrate
(Co(NO_(3))_(2),182.94(g)/(m)ol)? Assume you will need
100mLof working stock. Target working stock] in ppm (mg/L): Mass of cobalt nitrate needed for working stock: Mass of cobalt nitrate weighed out: [master stock] in ppm
(m(g)/(L)):Number of 1:10 dilutions to go from master stock to working stock: Bonus (
+5pts): Assuming
0.3096gof cobalt nitrate was weighed out, fill in the table with the actual concentrations of the standards, if they were made in
50mLvolumetric flasks.