Home /
Expert Answers /
Chemistry /
buffer-preparation-acid-base-reactions-tr-use-the-references-to-act-an-aqueous-solution-contains-0-pa869
(Solved): Buffer Preparation: Acid-Base Reactions TR Use the References to act An aqueous solution contains 0. ...
Buffer Preparation: Acid-Base Reactions
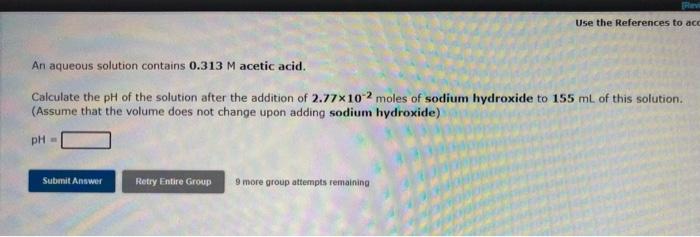



TR Use the References to act An aqueous solution contains 0.313 M acetic acid. Calculate the pH of the solution after the addition of 2.77x102 moles of sodium hydroxide to 155 ml of this solution. (Assume that the volume does not change upon adding sodium hydroxide) pH = Submit Answer Retry Entire Group 9 more group attempts remaining
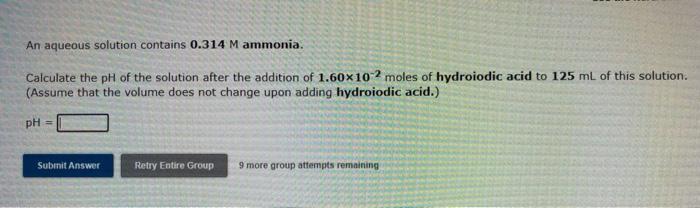
An aqueous solution contains 0.314 M ammonia. Calculate the pH of the solution after the addition of 1.60x102 moles of hydroiodic acid to 125 mL of this solution. (Assume that the volume does not change upon adding hydroiodic acid.) pH = Submit Answer Retry Entire Group 9 more group attempts remaining
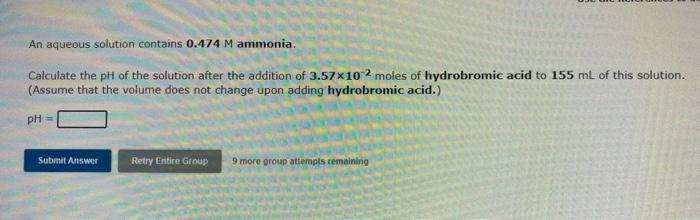
An aqueous solution contains 0.474 M ammonia. Calculate the pH of the solution after the addition of 3.57x10-2 moles of hydrobromic acid to 155 mL of this solution. (Assume that the volume does not change upon adding hydrobromic acid.) pH = Submit Answer Retry Entire Group 9 more group attempts remaining