Home /
Expert Answers /
Chemistry /
butane-gas-c4h10-is-sold-to-campers-as-bottled-fuel-its-density-at-25-degrees-celsius-and-1-atm-i-pa674
(Solved): Butane gas, C4H10, is sold to campers as bottled fuel. Its density at 25 degrees Celsius and 1 atm i ...
Butane gas, C4H10, is sold to campers as bottled fuel. Its density at 25 degrees Celsius and 1 atm is 2.38 grams per liter. what volume of butane gas at 25 degrees Celsius and 1 atm is required to heat one gallon of water from 25 degrees Celsius to 98 degrees Celsius? The reaction for combustion of butane (Delta H= -125.6 kJ/mol) is C4H10(g)+ 13/2 O2(g) ---> 4CO2(g) +5H20(g)
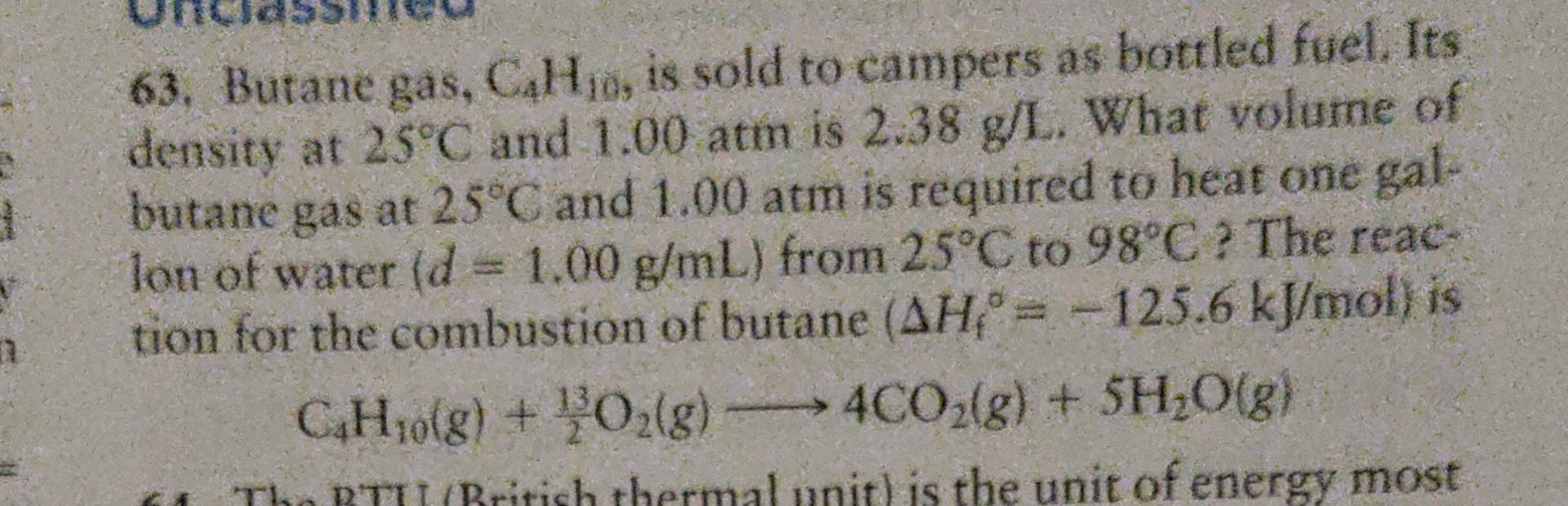
63. Butane gas, \( \mathrm{C}_{4} \mathrm{H}_{10} \), is sold to campers as bottled fuel. Its density at \( 25^{\circ} \mathrm{C} \) and \( 1.00 \mathrm{~atm} \) is \( 2.38 \mathrm{~g} / \mathrm{L} \). What volume of butane gas at \( 25^{\circ} \mathrm{C} \) and \( 1.00 \mathrm{~atm} \) is required to heat one gallon of water \( (d=1.00 \mathrm{~g} / \mathrm{mL}) \) from \( 25^{\circ} \mathrm{C} \) to \( 98^{\circ} \mathrm{C} \) ? The reaction for the combustion of butane \( \left(\Delta H_{\mathrm{f}}{ }^{\circ}=-125.6 \mathrm{~kJ} / \mathrm{mol}\right) \) is \[ \mathrm{C}_{4} \mathrm{H}_{10}(g)+\frac{13}{2} \mathrm{O}_{2}(g) \longrightarrow 4 \mathrm{CO}_{2}(g)+5 \mathrm{H}_{2} \mathrm{O}(g) \]
Expert Answer
The combustion of butane C4H10 is C4H10(g)+ 13/2 O2----->4CO2(g)+5H2O(g) The standard heat of reaction = sum of standard heat of formation of products- sum of standard heat of formatino of reactants= 4*standard heat of formation of CO2+5* standard he